TO THE EDITOR: A transfusion reaction (TR) is any untoward event that occurs during or after blood transfusion and is not related to the patient’s underlying illness. It has been estimated that about 10% of all transfusions carry the risk of an adverse event [1]. Both, infectious and non-infectious TRs are associated with significant mortality and morbidity [2]. The frequency of TR is estimated to be 0.5 to 2.9 per 1,000 blood units [3456]. However, the actual TR incidence is frequently underestimated; therefore, every hospital should have a hemovigilance program aimed at effectively reporting and analyzing TR in order to improve transfusion patient safety [5].
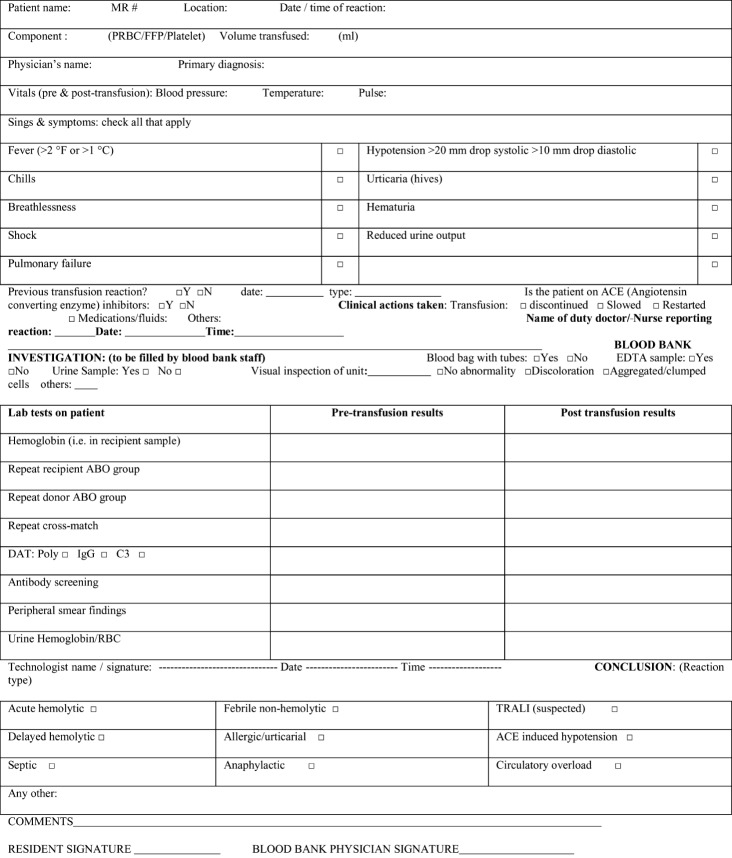
Here we describe a clinical audit conducted at the Isratul Ebad Khan Institute of Blood Diseases (IEKIBD), Dow University Hospital, Pakistan. The audit was undertaken as an institutional effort towards hemovigilance with the aim of observing the frequency of reported TRs and assessing the effects of measures taken to avoid TR under-reporting. A standard TR investigation protocol was used (Fig. 1) and TRs were classified according to standard AABB definitions.
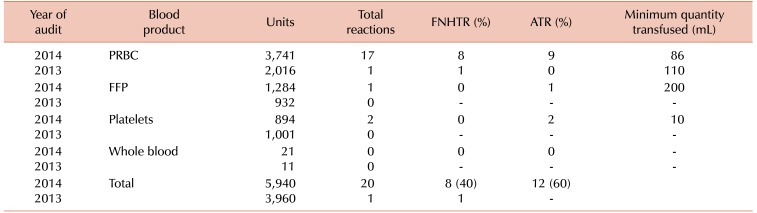
From January to December 2013, approximately 3,960 blood units were released for transfusion. Out of these blood units, only one febrile non-hemolytic transfusion reaction (FNHTR) was reported. The estimated rate of TR was found to be 0.2 per 1,000 blood units administered (Table 1). This rate of TR was found to be low when compared to local and international studies, where the rate of TRs per 1,000 units was 0.93 to 1.16 [34] and 0.4 to 2.9 [56], respectively. To investigate the root cause of this presumed under-reporting of TR, we designed and distributed an in-house questionnaire regarding the signs and symptoms of TRs to evaluate the ability of medical and nursing staff to recognize and report any adverse TR. An open-ended question was included about the reasons for not reporting a TR. On evaluation, it was found that about 73% of medical and nursing staff were well aware of the signs and symptoms of TR. Reasons for TR under-reporting were found to be multifactorial and mainly included a lack of easy accessibility of TR forms in different hospital units, a lack of awareness about existing TR reporting systems among newly inducted interns and residents, and the irrational use of transfusion premedication (antihistamines and NSAIDs) without knowing the patient’s previous history of TR.
The following steps were then taken to improve overall TR reporting:
1. Easy accessibility of TR forms: To improve the accessibility of TR forms, we printed them on the reverse side of the cross-match product releasing slip, so that every unit of blood leaving the blood bank would automatically be accompanied by a TR form.
2. TR Awareness sessions: Interactive sessions with nursing/medical staff were carried out to familiarize them with the institution’s existing system for reporting TR. Information flyers were also distributed highlighting the cardinal signs and symptoms of TR to enhance staff’s ability to identify TRs.
3. Discouraging transfusion pre-medication: Based on the results of a literature search [7], the use of transfusion premedication was discouraged in patients receiving a transfusion for the first time as it could mask the likely occurrence of FNHTR or allergic transfusion reaction (ATR), as well as giving the potential to miss a more severe reaction like acute hemolytic transfusion reaction (HTR) or a septic reaction.
A post-audit analysis was conducted from January to December 2014. Overall, 5,940 blood products were transfused and 20 TRs were reported. The rate of reported TRs was 3.4 per 1,000 blood products administered. Of the 20 TRs, 16 occurred in women and 4 in men. The median patient age was 40 years (±20 years). The frequency of TR was highest for PRBC (4.5/1,000) transfusion, followed by platelets (2.2/1,000), and fresh frozen plasma (0.3/1,000). The spectrum of adverse TRs noted with different blood products is shown in Table 1. ATR was the most frequent adverse event, accounting for 60% of all events, followed by FNHTR, which accounted for 40%. Medical and nursing staff equally reported these reactions. No transfusion reaction occurred because of clerical errors, including ABO-mismatched component transfusion. Following the implementation of new strategies, the rate of TR was increased from 0.2 to 3.4 per 1,000 transfusions.
In this study, the result of the initial audit was consistent with the assumption of under-reporting of TR. The post-audit rate of TR in our study was the same as the ones in the studies from countries like the Netherlands and Namibia, which were 3.3 and 3.4 per 1,000 units, respectively [89]. However, it was higher than the results of previous studies from Pakistan, which were 0.8 and 1.16 per 1,000 units [34]. Most of the authors of previous studies identified an inability to recognize TRs as the most common reason for the under-reporting of the events [10]. However, in this study we found that irrational use of transfusion premedication, unavailability of TR forms, and limited information on the institutional TR reporting system among health care workers were common reasons for TR under-reporting. The efficacy of pre-transfusion medication for the prevention of acute TRs like FNHTR and ATR have been assessed in few studies and these showed that prophylactic medication does not reduce the incidence of FNHTRs or ATRs [7]. Heddle et al. [11] found that the use of antipyretics prevented fever but not other symptoms of FNHTR such as chills, cold, and discomfort. Anti-pyretic medication can also mask fever as an initial sign of acute HTR or septic reaction, thereby delaying the critical management of these more hazardous TRs. Besides the medical consequences, it has been estimated that transfusion premedication costs more than $40,000 annually [12]. Based on these findings, the irrational use of transfusion premedication was strictly discouraged and this actually enhanced the TR frequency in our setup. None of the previous studies have reported transfusion premedication as the cause of under reporting of TR.
This is the first complete audit report on TRs reported from a public sector hospital in Pakistan. We observed an increased frequency of reported TRs of 3.4 adverse reactions per 1,000 transfusions after implementation of user-friendly TR forms, along with discouragement of transfusion pre-medication administration where it was not required. Regular audit of TRs as a quality indicator helped our institute to develop effective reporting strategies and increase TR awareness among physicians.
Acknowledgments
Anila Rashid collected data, statistically analyzed, and drafted the manuscript. Saima Minhas reviewed the manuscript and provided new ideas to incorporate into the paper. Bipin Nepal and Nadeem Nusrat helped in collecting the data. Mohammad Akbar Aga participated in study design, conception and drafting of manuscript. All authors read and approved the final manuscript.
Go to : 
Notes
Authors' Disclosures of Potential Conflicts of Interest: No potential conflicts of interest relevant to this article were reported.
Go to : 
References
1. Rabeya Y, Abdul-Kahar AH, Leong CF. An audit of reported acute transfusion reactions in Universiti Kebangsaan Malaysia Medical Centre. Malays J Pathol. 2011; 33:25–29. PMID: 21874748.
2. Sazama K. Reports of 355 transfusion-associated deaths: 1976 through 1985. Transfusion. 1990; 30:583–590. PMID: 2402771.

3. Karim F, Moiz B, Shamsuddin N, Naz S, Khurshid M. Root cause analysis of non-infectious transfusion complications and the lessons learnt. Transfus Apher Sci. 2014; 50:111–117. PMID: 24239270.

4. Khalid S, Usman M, Khurshid M. Acute transfusion reactions encountered in patients at a tertiary care center. J Pak Med Assoc. 2010; 60:832–836. PMID: 21381614.
5. Kumar P, Thapliyal R, Coshic P, Chatterjee K. Retrospective evaluation of adverse transfusion reactions following blood product transfusion from a tertiary care hospital: A preliminary step towards hemovigilance. Asian J Transfus Sci. 2013; 7:109–115. PMID: 24014939.

6. Kubaski F, Lunkes DS, Onsten TG. Reporting of transfusion reactions in a hospital in Brazil. Blood Transfus. 2013; 11:151. PMID: 22790274.
7. Tobian AA, King KE, Ness PM. Transfusion premedications: a growing practice not based on evidence. Transfusion. 2007; 47:1089–1096. PMID: 17524101.

8. Wiersum-Osselton JC, van Tilborgh-de Jong AJ, Zijlker-Jansen PY, et al. Variation between hospitals in rates of reported transfusion reactions: is a high reporting rate an indicator of safer transfusion? Vox Sang. 2013; 104:127–134. PMID: 22892067.

9. Meza BP, Lohrke B, Wilkinson R, et al. Estimation of the prevalence and rate of acute transfusion reactions occurring in Windhoek, Namibia. Blood Transfus. 2014; 12:352–361. PMID: 24333079.
10. Basavaraju SV, Lohrke B, Pitman JP, et al. Knowledge and barriers related to reporting of acute transfusion reactions among healthcare workers in Namibia. Transfus Med. 2013; 23:367–369. PMID: 23841708.

11. Heddle NM, Klama LN, Griffith L, Roberts R, Shukla G, Kelton JG. A prospective study to identify the risk factors associated with acute reactions to platelet and red cell transfusions. Transfusion. 1993; 33:794–797. PMID: 8236418.

12. Sanders RP, Maddirala SD, Geiger TL, et al. Premedication with acetaminophen or diphenhydramine for transfusion with leucoreduced blood products in children. Br J Haematol. 2005; 130:781–787. PMID: 16115137.

Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download