Abstract
Background
Characterization of the ABO blood group at the phenotype and genotype levels is clinically essential for transfusion, forensics, and population studies. This study elucidated ABO phenotypes and genotypes, and performed an evaluation of their distribution in individuals from the western region of Saudi Arabia.
Methods
One-hundred and seven samples underwent standard serological techniques for ABO blood group phenotype analysis. ABO alleles and genotypes were identified using multiplex polymerase chain reaction, and electrophoretic analysis was performed to evaluate the highly polymorphic ABO locus.
Results
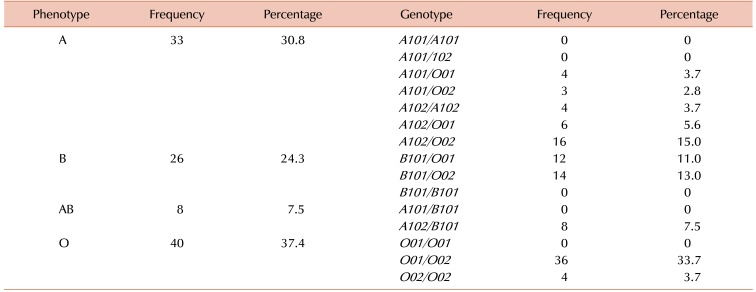
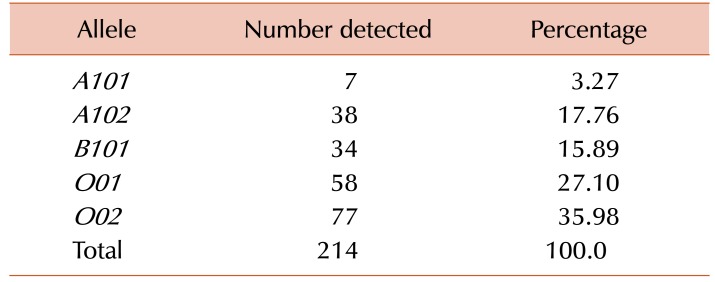
A phenotype distribution of 37.4%, 30.8%, 24.3%, and 7.5% was found for blood groups O, A, B, and AB respectively in our study cohort. Genotype analysis identified 10 genotype combinations with the O01/O02 and A102/O02 genotypes being the most frequent with frequencies of 33.6% and 14.95%, respectively. Common genotypes such as A101/A101, A101/A102, A101/B101, B101/B101, and O01/O01 were not detected. Similarly, the rare genotypes, cis-AB01/O02, cis-AB01/O01, and cis-AB01/A102 were not found in our cohort. The most frequently observed allele was O02 (35.98%) followed by the A102 allele (17.76%). Furthermore, our findings are discussed in reference to ABO allele and genotype frequencies found in other ethnic groups.
The ABO gene, which is located on chromosome 9, has seven exons and is over 18 kilobases (kb) in length. The major coding region of ABO is found in exons 6 and 7 [1], and many ABO alleles have been described [2]. These alleles have minor differences in their genomic sequences, and most have emerged because of hybrid alleles, base insertions, deletions, or substitutions, or by splice site mutations [23]. Furthermore, the frequencies of these alleles differ among different populations and ethnicities. For example, the A101, O01, B101, A102, and O02 alleles are common in Asian populations [14]. Alleles such as O04 and A205 are found in the Han Chinese, and allele O06 is found in the Japanese [56]. Rare alleles such as cis-AB01, cis-AB02, and cis-AB06 are more frequently encountered in the Japanese, Korean, and Chinese populations [578].
The ABO locus has three main phenotypes, A, B, and O, with the combination of glucosyltransferases encoded by different alleles determining the A, B, AB, or O blood group phenotype [910]. Each phenotype can be distinguished serologically using anti-A and anti-B antisera generated by polyclonal or monoclonal techniques. These serological tests are important for matching the blood groups of acceptors and donors and not only enable the determination of additional subgroups, but also facilitate differentiation between weak A and B antigens [1112]. From an application perspective, allele and genotype studies of ABO are of primary importance in paternal discrepancy, forensics, organ transplantation, and population studies [11]. A number of strategies based on polymerase chain reaction (PCR) have been developed for ABO genotyping including restriction fragment-length polymorphism (RFLP) analysis and allele-specific PCR [13], single-strand conformation polymorphism analysis [14], amplified product length polymorphism (APLP), and sequencing [15]. To date, there has been no study in the western region of Saudi Arabia using these more current genotyping approaches. Therefore, the purpose of this study was to determine and evaluate the frequency and distribution of ABO phenotypes and their alleles using specific primers by multiplex PCR.
This prospective study was approved by the ethical committee of the Faculty of Medicine, King Abdulaziz University Hospital at Jeddah (Saudi Arabia). Peripheral blood was obtained with informed consent from 107 participants. ABO phenotypes were serologically determined with anti-A and anti-B antibodies using an AutoVue Innova (Ortho Clinical Diagnostics, Raritan, NJ, USA).
Genomic deoxyribonucleic acid (DNA) from blood was extracted using the QIAamp DNA kit (Qiagen, Hilden, Germany). Briefly, 200 µL of sample was mixed with 20 µL of Qiagen protease in a microcentrifuge tube; 200 µL of buffer AL was added followed by thorough mixing and incubation at 56℃ for 10 min. Afterwards, 200 µL of absolute ethanol was added to the lysate, followed by gentle vortexing for proper mixing. Next, the mixture was transferred to a mini spin column for centrifugation and washed three time using buffer AWI before a final elution with 50 µL of AE buffer. Extracted DNA was quantified using a NanoDrop spectrophotometer (Thermo Fisher Scientific Inc., Wilmington, DE, USA). The method followed the procedure used by Muro et al. [16].
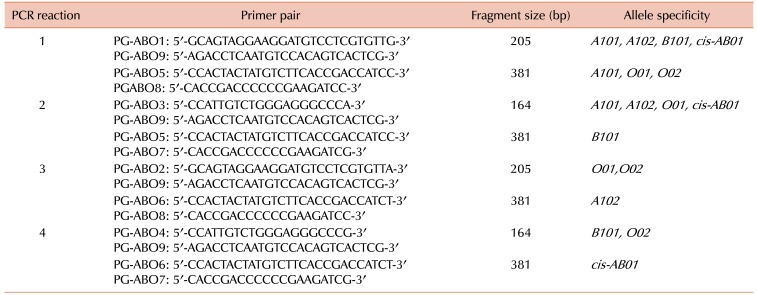
Primers used for PCR are listed in Table 1. The genotyping approach for this study was used previously in several studies [1718]. Four separate PCR reactions were performed using different sets of primers in each sample. Each 20 µL reaction contained 2 µL DNA mixed with 1 µL of each allele-specific primer (10 pmol), 10 µL master mix (2X), and 7 µL nuclease free water. PCR was performed in a thermal cycler (Applied Biosystems, Wilmington, DE, USA). The cycling conditions followed an initial denaturation at 95℃ for 3 min, followed by 35 cycles of denaturation at 95℃ for 40 s, annealing at 56℃ for 40 s, and elongation at 72℃ for 40 s, followed with a final elongation at 72℃ for 5 min. PCR products were separated by electrophoresis on 3% agarose gels containing ethidium bromide at 40 mV for 20 min followed by 70 mV for 30–40 min. A 100 base pair (bp) DNA ladder was used as a molecular marker (Invitrogen, Wilmington, DE, USA). The gel was visualized using a G-BOX UV transilluminator (Syngene, Cambridge, UK).
We found that serological ABO typing and phenotypes of study participants inferred from genotypes were concordant with an expected distribution between the A (N=33; 30.84%), B (N=26; 24.30%), AB (N=8; 7.48%), and O (N=40; 37.38%) blood groups.
Out of 21 possible ABO genotypes, 10 genotypes were observed in our study group (Table 2). The O01/O02 genotype was the most frequent accounting for 33.6% of participants followed by the A102/O02 genotype that was found in 14.9% of participants. In addition, we found that frequencies of the B101/O02, B101/O01, A102/B101, and A102/O01 genotypes were 13.1%, 11.2%, 7.5%, and 5.6%, respectively. Genotypes such as A101/A101, A101/A102, A101/B101, O01/O01, and B101/B101 as well as rare genotypes including cis-AB01/O02, cis-AB01/O01, and cis-AB01/A102, were not be detected in our cohort. We found that eight individuals were homozygous for either A102/A102 (N=4) or O02/O02 (N=4), while the remaining 99 individuals were heterozygous.
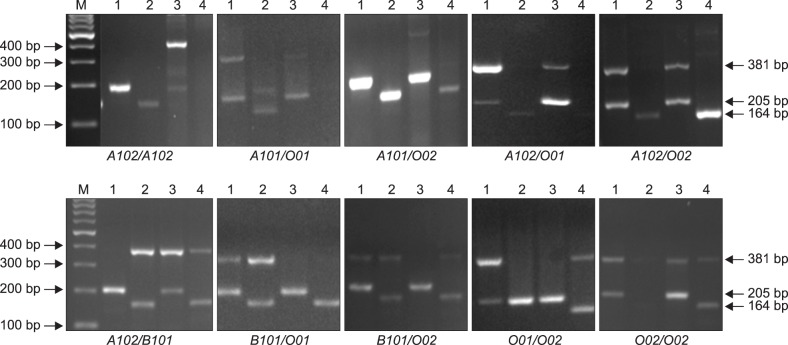
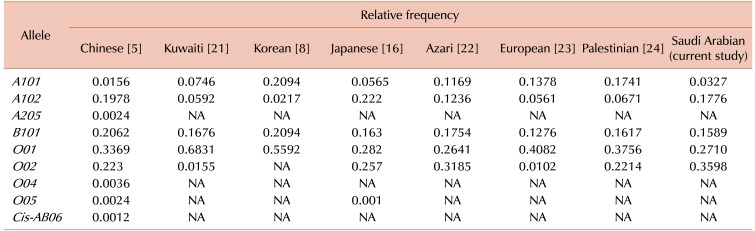
Primers used to amplify the six alleles of ABO are shown in Table 1. The frequency distributions of ABO phenotypes, genotypes, and alleles are summarized in Table 2 and Table 3. Our allele distribution consisted of A101 (N=7), A102 (N=38), B101 (N=34), O01 (N=58), and O02 (N=77). We found that O02 was the most abundant allele in our study cohort accounting for 36.0% of all alleles, while the A102 allele had a frequency of 17.8%. The frequencies of the B101 and O01 alleles were 15.9% and 27.1% respectively, and the A101 allele had the lowest frequency at 3.27%. We found that our western Saudi population had an increased frequency of the O02 allele compare to the O01 allele. Fig. 1 shows the electrophoretic patterns of the 10 recognized genotypes, while Table 4 provides a comparison of allele frequencies among different populations.
We found in our cohort from the western region of Saudi Arabia phenotype frequencies of 30.8%, 24.3%, 7.5%, and 37.4% for the A, B, AB, and O blood groups, respectively. Previous studies performed in other Saudi populations found different frequencies of 34.2%, 24.8%, 2.5%, and 38.5% for the A, B, AB, and O blood groups, respectively [19]. For all studies including the present one, blood group O has the highest frequency followed by A, B, and AB. This variation in ABO phenotype found between different Saudi populations is expected among ethnic groups [20]. For example, in the United Kingdom, blood group B has a low frequency (10%), whereas in India it is 18.8% [4].
We found 10 ABO genotypes in our cohort, of which O01/O02 was the most predominant found in 33.6% of individuals. These findings corroborate similar findings in a Han Chinese population where O01/O02 is the most frequently observed genotype (16.8%). In addition, we found that the frequency of the O02/O02 genotype in our Saudi population (3.7%) was comparable to that found in a Han Chinese population. Furthermore, and similar to our findings, another study did not find the A101/A101 genotype in their cohort [5]. However, our findings differed from those found in a Kuwaiti population where the homozygous O01/O01 genotype was detected in 47.0% of blood samples [21] whereas it has not been reported in a Saudi population [19]. In contrast, we found that the A102/O02 genotype was present in 14.9% of blood samples which was absent in the Kuwaiti population. Rare genotypes such as cis-AB01/O02, cis-AB01/O01, and cis-AB01/A102 were conspicuously absent in the current study with these latter alleles being detected among 13 different genotypes in the Korean population [8]. In addition, the cis-AB01 genotype has been reported in Japanese and Chinese populations [56]. The absence of these genotypes cis-AB01/O02, cis-AB01/O01 and cis-AB01/ A102 in studies from the Middle East indicates that the Middle Eastern population differs considerably from the Chinese, Korean, and Japanese populations in respect to these rare genotypes.
Of the six possible alleles, we found five alleles of which the most frequent allele was O02 with a frequency of 0.3598 followed by the O01 allele with a frequency of 0.271. A comparable study on ABO blood group genotypes in a Chinese population found that the O01 allele was the most frequent with a frequency of 0.3369 followed by the O02 allele. In addition, alleles A102 and B101 with frequencies of 0.1978 and 0.2062 in our cohort were comparable to frequencies of 0.1776 and 0.1589 respectively, in the Chinese cohort [5].
As anticipated, the frequency of various alleles in different populations around the world can differ widely because of ethnicity (demonstrated in Table 4). This variation has been widely observed in many studies [56821222324]. In the majority of populations examined, the A101 and A102 alleles are common, whereas the O02 allele is predominant in the Saudi population. In comparison, the O01 and O02 alleles are frequent in Han Chinese, Kuwaiti, and other populations with varying frequencies [521]. Similar differences in allele frequencies are found in the European, Japanese, German, Kuwaiti, and Indian populations [462123].
In conclusion, the present study reports the distribution of ABO phenotypes and genotypes in a group of individuals from the western region of Saudi Arabia. Using allele-specific multiplex PCR, 10 ABO genotypes were identified. In addition, this pilot study identified five alleles A101, A102, B101, O01, and O02, which were found at different frequencies in our cohort, and we performed a comparative assessment to other studies. Future plans include analyzing a larger number of samples to obtain a more comprehensive understanding of the ABO genotype pattern in Saudi Arabia.
ACKNOWLEDGEMENTS
The authors thank the King Fahd Medical Research Center for providing research facilities for experiments.
ABOM and SIH performed the research; ABOM and QA designed the research study; MZA, ABOM, SIH, and AH analyzed the data; WA, MZA, ABOM, AH, and GAD authenticated the data and prepared the manuscript.
References
1. Chen YJ, Chen PS, Liu HM, et al. Novel polymorphisms in exons 6 and 7 of A/B alleles detected by polymerase chain reaction-single strand conformation polymorphism. Vox Sang. 2006; 90:119–127. PMID: 16430670.

2. Blumenfeld OO, Patnaik SK. Allelic genes of blood group antigens: a source of human mutations and cSNPs documented in the Blood Group Antigen Gene Mutation Database. Hum Mutat. 2004; 23:8–16. PMID: 14695527.

3. Olsson ML, Chester MA. Polymorphism and recombination events at the ABO locus: a major challenge for genomic ABO blood grouping strategies. Transfus Med. 2001; 11:295–313. PMID: 11532186.

4. Ray S, Gorakshakar AC, Vasantha K, Nadkarni A, Italia Y, Ghosh K. Molecular genotyping of ABO blood groups in some population groups from India. Indian J Med Res. 2014; 139:105–111. PMID: 24604045.
5. Zhu F, Tao S, Xu X, et al. Distribution of ABO blood group allele and identification of three novel alleles in the Chinese Han population. Vox Sang. 2010; 98:554–559. PMID: 20003128.

6. Maeda K, Nakamura S, Murakami C, et al. ABO genotyping by TaqMan assay and allele frequencies in a Japanese population. Leg Med (Tokyo). 2013; 15:57–60. PMID: 23067801.

7. Cho D, Shin MG, Yazer MH, et al. The genetic and phenotypic basis of blood group A subtypes in Koreans. Transfus Med. 2005; 15:329–334. PMID: 16101812.

8. Kang SH, Fukumori Y, Ohnoki S, et al. Distribution of ABO genotypes and allele frequencies in a Korean population. Jpn J Hum Genet. 1997; 42:331–335. PMID: 9290258.

9. Landsteiner K. Agglutination phenomena of normal human blood. Wien Klin Wochenschr. 2001; 113:768–769. PMID: 11732110.
10. Crow JF. Felix Bernstein and the first human marker locus. Genetics. 1993; 133:4–7. PMID: 8417988.

11. Olsson ML, Chester MA. Polymorphisms at the ABO locus in subgroup A individuals. Transfusion. 1996; 36:309–313. PMID: 8623129.

12. Yip SP. Single-tube multiplex PCR-SSCP analysis distinguishes 7 common ABO alleles and readily identifies new alleles. Blood. 2000; 95:1487–1492. PMID: 10666229.

13. Fukumori Y, Ohnoki S, Shibata H, Yamaguchi H, Nishimukai H. Genotyping of ABO blood groups by PCR and RFLP analysis of 5 nucleotide positions. Int J Legal Med. 1995; 107:179–182. PMID: 7599092.

14. Lee JC, Hsieh HM, Teng HF, Lo SC, Linacre A, Tsai LC. ABO genotyping by single strand conformation polymorphism--using CE. Electrophoresis. 2009; 30:2544–2548. PMID: 19639575.
15. Huh JY, Park G, Jang SJ, Moon DS, Park YJ. A rapid long PCR-direct sequencing analysis for ABO genotyping. Ann Clin Lab Sci. 2011; 41:340–345. PMID: 22166503.
16. Muro T, Fujihara J, Imamura S, et al. Determination of ABO genotypes by real-time PCR using allele-specific primers. Leg Med (Tokyo). 2012; 14:47–50. PMID: 22177907.

17. Yaku H, Yukimasa T, Nakano S, Sugimoto N, Oka H. Design of allele-specific primers and detection of the human ABO genotyping to avoid the pseudopositive problem. Electrophoresis. 2008; 29:4130–4140. PMID: 18991264.

18. Honda K, Nakamura T, Tanaka E, Yamazaki K, Tun Z, Misawa S. Graphic SSCP analysis of ABO genotypes for forensic application. Int Congr Ser. 2004; 1261:586–588.

19. Al Ghamdi AST. Association between ABO blood groups and severity of chronic periodontitis. JKAU Med Sci. 2009; 16:31–41.
20. Seltsam A, Hallensleben M, Kollmann A, Blasczyk R. The nature of diversity and diversification at the ABO locus. Blood. 2003; 102:3035–3042. PMID: 12829588.

21. El-Zawahri MM, Luqmani YA. Molecular genotyping and frequencies of A1, A2, B, O1 and O2 alleles of the ABO blood group system in a Kuwaiti population. Int J Hematol. 2008; 87:303–309. PMID: 18247104.
22. Nojavan M, Shamsasenjan K, Movassaghpour AA, Akbarzadehlaleh P, Torabi SE, Ghojazadeh M. Allelic prevalence of ABO blood group genes in Iranian Azari population. Bioimpacts. 2012; 2:207–212. PMID: 23678461.
23. Nishimukai H, Fukumori Y, Okiura T, et al. Genotyping of the ABO blood group system: analysis of nucleotide position 802 by PCR-RFLP and the distribution of ABO genotypes in a German population. Int J Legal Med. 1996; 109:90–93. PMID: 8912054.

24. Saqer LS, Sharif FA. Allele and genotype frequencies of the ABO blood group system in a palestinian population. Asian J Pharm Nurs Med Sci. 2013; 1:98–103.
Fig. 1
Electrophoretic pattern of the 10 identified ABO group genotypes in a Saudi population using allele-specific multiplex polymerase chain reaction (PCR). The numbers on top of each gel are the PCR reaction tube number. Column M shows a 100 base pair (bp) DNA ladder.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download