Abstract
Background
Kasabach-Merritt syndrome (KMS) is a rare but life-threatening illness. The purpose of this study is to report our single-center experience with KMS.
Methods
We reviewed the medical records of 13 patients who were diagnosed with KMS between 1997 and 2012 at Samsung Medical Center. Treatment response was defined as follows: 1) hematologic complete response (HCR) – platelet count >130×109/L without transfusion; 2) clinical complete response (CCR) – complete tumor disappearance or small residual vascular tumor displaying lack of proliferation for at least 6 months after treatment discontinuation.
Results
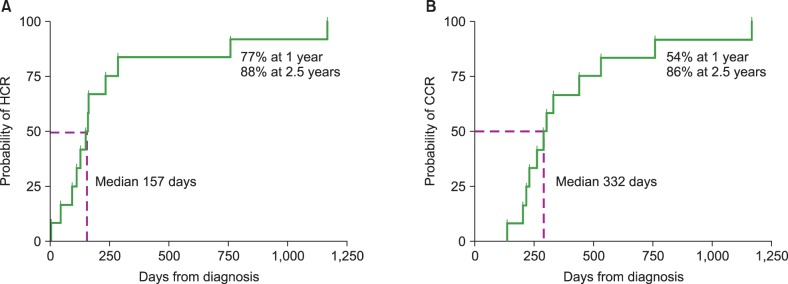
Participants included 7 male and 6 female patients. The median initial hemoglobin levels and platelet counts were 9.7 g/dL (range, 6.6–11.6 g/dL) and 11×109/L (range, 3–38×109/L), respectively. Twelve patients received corticosteroid and interferon-alpha as initial treatment, and the remaining patient received propranolol instead of corticosteroid. Two patients with unsatisfactory response to the initial treatment received weekly vincristine. Successful discontinuation of medication was possible at a median of 301 days (range, 137–579) in all patients except one who was lost to follow-up. The median times to achieve HCR and CCR were 157 days and 332 days, respectively. The probabilities of achieving HCR and CCR were 77% and 54% at 1 year, and 88% and 86% at 2.5 years, respectively.
Kasabach-Merritt syndrome (KMS) is characterized by a giant hemangioma with thrombocytopenia, microangiopathic hemolytic anemia, and consumptive coagulopathy that can lead to life-threatening bleeding [1]. KMS usually develops in infancy, and it is usually associated with kaposiform hemangioendothelioma and tufted angioma, rather than the classic hemangioma of infancy [2]. Untreated KMS can be life threatening because of the involvement of multiple organs and the unresponsiveness of the condition to treatment, and it has shown relatively high mortality rates of up to 30% [3]. Since the first report of KMS in 1940 by Kasabach and Merritt [4], various types of treatment, including medical treatment, vascular embolization, radiation therapy, and surgery, have been attempted to achieve hematologic and clinical response [56789]. A number of treatments for KMS have been reported, but none have been uniformly effective. In addition, estimates of the effectiveness of different treatment modalities are limited because KMS is a rare condition and most previous reports have been small case series. As a result, standard regimens and definitive guidelines for the treatment of KMS have not yet been established.
Herein, we report our single-center experience of patients with KMS who received combined medical treatments without surgical intervention or radiotherapy.
A retrospective review of the medical records was performed for 13 patients who were diagnosed with KMS between January 1997 and December 2012 at Samsung Medical Center. The diagnosis of KMS was defined as superficial or visceral hemangioma with thrombocytopenia and/or abnormal hematologic data suggesting disseminated intravascular coagulation (DIC). Hemangioma was confirmed by gross appearance in cases when it was located externally and by imaging studies such as ultrasonography, blood pool scan, and magnetic resonance imaging when it was located internally. Biopsy was performed in only one case, but the procedure was not routinely performed for the diagnostic confirmation of hemangioma.
All patients were initially treated with combined medical therapy composed of oral prednisolone or intravenous methylprednisolone (2 mg/kg/day), oral propranolol (2 mg/kg/day), and interferon-alpha (IFNα) (100,000 IU/kg/day). If the platelet count did not increase substantially by 2 weeks, or if significant complications occurred, weekly vincristine (0.05 mg/kg for <1 year or 1.5 mg/m2 for ≥1 year) was considered. Details of treatment are provided in Table 1. Treatment outcome was classified as hematologic complete response (HCR) if the patient’s platelet count exceeded 130×109/L without transfusion support and as clinical complete response (CCR) if the patient showed either complete disappearance of the tumor or had a small residual vascular tumor that displayed lack of proliferation for at least 6 months after discontinuation of treatment. For statistical analysis, the Kaplan-Meier method was used to estimate HCR and CCR. The institutional review board (IRB) of Samsung Medical Center approved this retrospective study and waived the requirement for informed consent (IRB No. 2015-01-063).
Clinical and hematologic data, treatment, and final outcomes are summarized in Table 2. The total number of patients was 13 (7 male and 6 female) and the median age at diagnosis was 30 days (range, 0 to 593). Clinically, the most common location of hemangioma was the extremities (N=7), followed by the trunk (N=2), retroperitoneum (N=2), neck (N=1), and spleen (N=1). No patients had obstructive symptoms such as biliary tract obstruction or urinary retention. Histologic confirmation was performed in one patient, yielding a diagnosis of lobular capillary hemangioma.
The median platelet count at diagnosis was 11×109/L (range, 3 to 38). The median fibrinogen level was 123 mg/dL (range, 47 to 323) and the median D-dimer level was 14 g/mL (range, 1 to 51). The median hemoglobin value at diagnosis was 9.7 g/dL (range, 6.6 to 11.6).
The details of treatment are listed in Table 1. Eleven of 13 patients (cases 1 to 11; 84.6%) received prednisolone (or methylprednisolone) and IFNα as first-line treatment; vincristine was added for 2 patients (cases 5 and 6) who showed no response to the initial treatment after 2 weeks. Among these 11 patients, 10 (90.9%) achieved HCR and CCR, and the remaining patient (case 4) was lost to follow-up. The length of steroid treatment varied according to the individual response and side effects, and the median duration was 52 days (range, 8 to 235). The steroid was generally maintained at the starting dose for up to 4 weeks and was then gradually withdrawn over the following 1–2 months. In some cases, low-dose steroid was maintained beyond 6 months to prevent disease recurrence or reactivation.
Case 12 received prednisolone and propranolol initially, and IFNα was added due to a poor response to the initial treatment. Prednisolone was tapered off after the achievement of HCR, and propranolol was maintained until CCR was reached. Case 13 received propranolol and IFNα as first-line therapy and eventually achieved HCR and CCR without steroid administration (Table 2).
Ultimately, all 13 patients received medical treatment without other intervention or surgery. The median times to achieve HCR and CCR were 157 days (range, 5 to 1,167) and 332 days (range, 137 to 1,167), respectively. The median treatment duration with any agent was 301 days (range, 137 to 579), while that with steroid was 52 days (range, 0 to 235) and that with IFNα was 159 days (range, 6 to 428). The probabilities of achieving HCR and CCR were 77% and 54% at 1 year, and 88% and 86% at 2.5 years, respectively (Fig. 1).
KMS is frequently associated with significant morbidity and mortality. Fatal complications of the syndrome are diverse, including hemorrhage, cardiac failure, and invasion of vital organs. Although different therapies for KMS have shown variable responses and outcomes [101112131415161718], the optimal treatment and consensus guidelines for KMS have not been established, a circumstance largely attributable to the rarity of the syndrome. Currently, various treatment options available for KMS include medical treatments, vascular embolization, radiation therapy, and surgery [56789]. Enjolras et al. [2] reported the risks associated with embolization treatment including ischemic damage, infarction of vital organs, worsening of hematological parameters, and the eventual formation of collateral vessels. As revealed by previous studies, radiation therapy may be associated with severe complications such as opportunistic infection, fibrosis of the lung, bone deformities, growth impairment, and the development of a secondary tumor, most of which are especially problematic in growing children compared to adults [192021]. On the other hand, surgical removal of a huge vascular lesion can lead to significant complications, including massive bleeding and tissue ischemia, which require urgent medical treatment [22]. In the present study, we demonstrated an excellent outcome for patients with KMS with medical treatments alone, with no patient experiencing life-threatening complications. Thus, we believe that most patients with KMS can be initially treated with medical treatment alone under circumstances in which close monitoring is warranted and rapid intervention can be provided whenever needed.
Corticosteroid has generally been used as the first-line treatment for KMS. However, the outcomes of steroid therapy have varied, ranging from no efficacy to significant regression of the lesion [82324]. IFNα has also been used for patients with steroid-resistant disease and has shown good overall outcomes [1525]. The majority of the patients (92.3%) in our study received steroid and IFNα concomitantly and showed excellent outcomes without grade 3 or 4 adverse effects, findings that justify the administration of these two agents in combination as a reasonable initial treatment approach.
Vincristine has recently emerged as a first-line treatment option or as a second-line treatment for steroid-resistant KMS. Haisley-Royster et al. retrospectively reviewed 15 patients with KMS and reported that 11 (74%) were successfully treated with a combination of corticosteroid and vincristine as first-line therapy [1]. Although the remaining patients (26%) relapsed, they were eventually treated successfully with a second course of 4 doses of weekly vincristine. Fahrtash et al. [26] reported that only 3 (42.9%) of 7 patients with KMS were successfully treated using vincristine alone, a finding that suggests the limitation of vincristine monotherapy as an initial treatment modality. In the present study, vincristine was administered to 2 patients who had poor responses to steroid and IFNα, one of whom achieved HCR and CCR, while the other was lost to follow-up before the treatment response could be assessed. Considering these findings together, vincristine might serve as a promising agent for patients with disease that is resistant to first-line treatment with steroid and/or IFNα. Given the proven efficacy of vincristine in some patients with KMS, further studies are needed to determine the clear benefit of vincristine as a first-line treatment in combination with other agents. However, the well-known and severe adverse effects of this agent, including peripheral neuropathy and severe ileus, should be closely monitored.
Propranolol is a nonselective beta-blocker that has been observed to bring about dramatic involution of hemangiomas with few significant complications [272829]. Hermans et al. [16] reported that a 6-week-old boy with KMS achieved remission after receiving upfront propranolol in combination with 4 doses of weekly vincristine. Chiu et al. [12] reported on the efficacy of upfront propranolol in 11 patients with kaposiform hemangioendothelioma and the related variant tufted angioma, among whom 6 were diagnosed with KMS; only 4 patients (36%) out of the entire group, including 2 patients with KMS, showed favorable responses to propranolol. Those who did not respond to propranolol required another agent for disease control, a finding that implies the limitation of propranolol monotherapy upon diagnosis of KMS. However, the role of propranolol either as an initial drug in combination with other agents or as a maintenance drug in KMS needs to be defined. With the awareness that long-term use of steroid and/or IFNα can cause various complications, ways to minimize the use of those agents should be sought whenever possible. In this context, our data suggest the beneficial role of propranolol, which was used in 2 patients, demonstrating that one patient could be managed with a relatively short duration of IFNα treatment (case 12) while the other was able to achieve CR with no use of steroid at all (case 13). Because of the current lack of sufficient evidence regarding the efficacy of propranolol in KMS, a large prospective study will be required prior to the routine incorporation of this relatively safe drug in treatment of the syndrome.
The present study is limited by its small sample size and retrospective design. In addition, patients in our cohort were treated with heterogeneous treatment regimens, while all of the patients received only medical treatments that were adjusted depending on the individual responses. However, this adaptive treatment strategy ultimately led to HCR and CCR in all of the patients who were compliant with the treatments. According to our data, we can predict that more than half of patients and more than three quarters of patients are able to achieve CCR and HCR at 1 year, respectively. These data also show that a significant proportion of patients require years of continued treatments, which necessitates the development of a well-designed protocol that could maximize treatment efficacy and minimize treatment-related toxicity, focusing not only on cure but also on quality of life in patients with KMS.
In conclusion, our data suggest that initial treatment with a two-drug combination (any two of steroid, IFNα, and propranolol) is a reasonable strategy for most patients with KMS to control the proliferation of a vascular tumor and its related complications. Individualized treatment adaptation according to response may be very important for the successful treatment of patients with KMS.
References
1. Haisley-Royster C, Enjolras O, Frieden IJ, et al. Kasabach-Merritt phenomenon: a retrospective study of treatment with vincristine. J Pediatr Hematol Oncol. 2002; 24:459–462. PMID: 12218593.

2. Enjolras O, Wassef M, Mazoyer E, et al. Infants with Kasabach-Merritt syndrome do not have "true" hemangiomas. J Pediatr. 1997; 130:631–640. PMID: 9108863.

3. el-Dessouky M, Azmy AF, Raine PA, Young DG. Kasabach-Merritt syndrome. J Pediatr Surg. 1988; 23:109–111. PMID: 3278084.

4. Kasabach HH, Merritt KK. Capillary hemangioma with extensive purpura: report of a case. Am J Dis Child. 1940; 59:1063–1070.
5. Garcia-Monaco R, Giachetti A, Peralta O, et al. Kaposiform hemangioendothelioma with Kasabach-Merritt phenomenon: successful treatment with embolization and vincristine in two newborns. J Vasc Interv Radiol. 2012; 23:417–422. PMID: 22365299.

6. Akyüz C, Emir S, Büyükpamukçu M, et al. Successful treatment with interferon alfa in infiltrating angiolipoma: a case presenting with Kasabach-Merritt syndrome. Arch Dis Child. 2003; 88:67–68. PMID: 12495967.
7. Abass K, Saad H, Kherala M, Abd-Elsayed AA. Successful treatment of kasabach-merritt syndrome with vincristine and surgery: a case report and review of literature. Cases J. 2008; 1:9. PMID: 18577262.

8. Jiang RS, Hu R. Successful treatment of Kasabach-Merritt syndrome arising from kaposiform hemangioendothelioma by systemic corticosteroid therapy and surgery. Int J Clin Oncol. 2012; 17:512–516. PMID: 21947597.

9. Shin HY, Ryu KH, Ahn HS. Stepwise multimodal approach in the treatment of Kasabach-Merritt syndrome. Pediatr Int. 2000; 42:620–624. PMID: 11192517.

10. Ryan C, Price V, John P, et al. Kasabach-Merritt phenomenon: a single centre experience. Eur J Haematol. 2010; 84:97–104. PMID: 19889011.

11. Yoon HS, Lee JH, Moon HN, Seo JJ, Im HJ, Goo HW. Successful treatment of retroperitoneal infantile hemangioendothelioma with Kasabach-Merritt syndrome using steroid, alpha-interferon, and vincristine. J Pediatr Hematol Oncol. 2009; 31:952–954. PMID: 19875968.
12. Chiu YE, Drolet BA, Blei F, et al. Variable response to propranolol treatment of kaposiform hemangioendothelioma, tufted angioma, and Kasabach-Merritt phenomenon. Pediatr Blood Cancer. 2012; 59:934–938. PMID: 22648868.

13. Hauer J, Graubner U, Konstantopoulos N, Schmidt S, Pfluger T, Schmid I. Effective treatment of kaposiform hemangioendotheliomas associated with Kasabach-Merritt phenomenon using four-drug regimen. Pediatr Blood Cancer. 2007; 49:852–854. PMID: 16411198.

14. Yasui N, Koh K, Kato M, et al. Kasabach-Merritt phenomenon: a report of 11 cases from a single institution. J Pediatr Hematol Oncol. 2013; 35:554–558. PMID: 23389504.
15. Léauté-Labrèze C, Dumas de la Roque E, Hubiche T, Boralevi F, Thambo JB, Taïeb A. Propranolol for severe hemangiomas of infancy. N Engl J Med. 2008; 358:2649–2651. PMID: 18550886.

16. Hermans DJ, van Beynum IM, van der Vijver RJ, Kool LJ, de Blaauw I, van der Vleuten CJ. Kaposiform hemangioendothelioma with Kasabach-Merritt syndrome: a new indication for propranolol treatment. J Pediatr Hematol Oncol. 2011; 33:e171–e173. PMID: 21516018.
17. Arunachalam P, Kumar VR, Swathi D. Kasabach-Merritt syndrome with large cutaneous vascular tumors. J Indian Assoc Pediatr Surg. 2012; 17:33–36. PMID: 22279364.

18. Su L, Wang D, Fan X. Comprehensive therapy for hemangioma presenting with Kasabach-Merritt syndrome in the maxillofacial region. J Oral Maxillofac Surg. 2015; 73:92–98. PMID: 25511959.

19. Lindberg S, Karlsson P, Arvidsson B, Holmberg E, Lunberg LM, Wallgren A. Cancer incidence after radiotherapy for skin haemangioma during infancy. Acta Oncol. 1995; 34:735–740. PMID: 7576739.

20. Alvarez-Mendoza A, Lourdes TS, Ridaura-Sanz C, Ruiz-Maldonado R. Histopathology of vascular lesions found in Kasabach-Merritt syndrome: review based on 13 cases. Pediatr Dev Pathol. 2000; 3:556–560. PMID: 11000333.

21. Saito M, Gunji Y, Kashii Y, et al. Refractory kaposiform hemangioendothelioma that expressed vascular endothelial growth factor receptor (VEGFR)-2 and VEGFR-3: a case report. J Pediatr Hematol Oncol. 2009; 31:194–197. PMID: 19262246.
22. Moura R, Sobreira LM, Bertanha M, et al. Kasabach-Merritt syndrome: clinical vs. surgical treatment. J Vasc Bras. 2014; 13:330–335.

23. Wang P, Zhou W, Tao L, Zhao N, Chen XW. Clinical analysis of Kasabach-Merritt syndrome in 17 neonates. BMC Pediatr. 2014; 14:146. PMID: 24920221.

24. Dresse MF, David M, Hume H, et al. Successful treatment of Kasabach-Merritt syndrome with prednisone and epsilonaminocaproic acid. Pediatr Hematol Oncol. 1991; 8:329–334. PMID: 1669958.

25. Peker E, Kirimi E, Tuncer O, Ceylan A, Oner AF. Brachial plexus paralysis due to giant cavernous hemangioma with Kasabach-Merritt syndrome: successful management with interferon alpha. Platelets. 2009; 20:603–605. PMID: 19929246.

26. Fahrtash F, McCahon E, Arbuckle S. Successful treatment of kaposiform hemangioendothelioma and tufted angioma with vincristine. J Pediatr Hematol Oncol. 2010; 32:506–510. PMID: 20523249.

27. Vergine G, Marsciani A, Pedini A, et al. Efficacy of propranolol treatment in thyroid dysfunction associated with severe infantile hepatic hemangioma. Horm Res Paediatr. 2012; 78:256–260. PMID: 22907522.

28. Zhan MK, Xie YD, Guo ZH, et al. Preliminary clinical study on the treatment of severe infantile hemangioma with high-dose propranolol in China. Zhonghua Zheng Xing Wai Ke Za Zhi. 2011; 27:166–169. PMID: 21837992.
29. Bonanno C, Paccanaro M, Fontanellilea A. Propranolol for severe hemangioma of infancy. J Cardiovasc Med (Hagerstown). 2011; 12:73. PMID: 21263236.

Fig. 1
Probabilities of hematologic complete response (HCR) and clinical complete response (CCR). (A) The median time to achieve HCR was 157 days. The probability of achieving HCR at 1 year and 2.5 years was 77% and 88%, respectively. (B) The median time to achieve CCR was 332 days. The probability of achieving CCR at 1 year and 2.5 years was 54% and 86%, respectively.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download