1. Kyle RA, Maldonado JE, Bayrd ED. Plasma cell leukemia. Report on 17 cases. Arch Intern Med. 1974; 133:813–818. PMID:
4821776.

2. Swerdlow SH, Campo E, Harris NL, editors. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon, France: IARC Press;2008.
3. Fernández de Larrea C, Kyle RA, Durie BG, et al. Plasma cell leukemia: consensus statement on diagnostic requirements, response criteria and treatment recommendations by the International Myeloma Working Group. Leukemia. 2013; 27:780–791. PMID:
23288300.

4. Cifola I, Lionetti M, Pinatel E, et al. Whole-exome sequencing of primary plasma cell leukemia discloses heterogeneous mutational patterns. Oncotarget. 2015; 6:17543–17558. PMID:
26046463.

5. Raj RS, Najeeb S, Aruna R, Pavithran K, Thomas M. Primary plasma cell leukemia occuring in the young. Indian J Cancer. 2003; 40:116–117. PMID:
14716116.
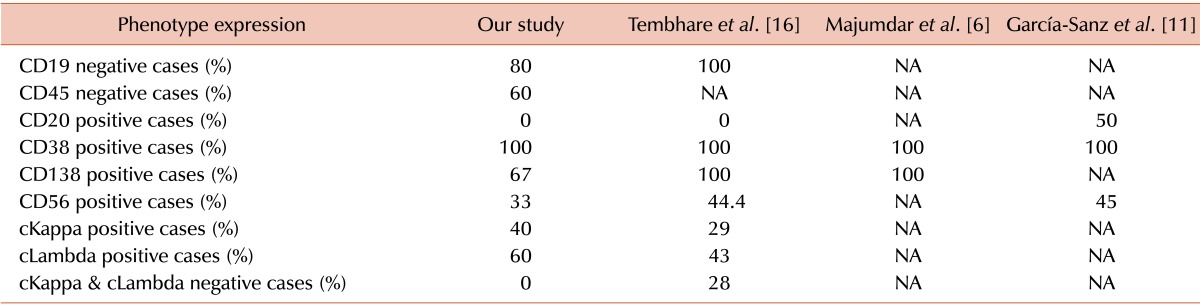
6. Majumdar N, Kumar R, Anand M, et al. Plasma cell leukemia-a study of 28 cases from India. Hematology. 2009; 14:198–203. PMID:
19635182.
7. Johnson MR, Del Carpio-Jayo D, Lin P, et al. Primary plasma cell leukemia: morphologic, immunophenotypic, and cytogenetic features of 4 cases treated with chemotherapy and stem cell transplantation. Ann Diagn Pathol. 2006; 10:263–268. PMID:
16979517.

8. Woodruff RK, Malpas JS, Paxton AM, Lister TA. Plasma cell leukemia (PCL): A report on 15 patients. Blood. 1978; 52:839–845. PMID:
687831.

9. Noel P, Kyle RA. Plasma cell leukemia: an evaluation of response to therapy. Am J Med. 1987; 83:1062–1068. PMID:
3503574.

10. Dimopoulos MA, Palumbo A, Delasalle KB, Alexanian R. Primary plasma cell leukaemia. Br J Haematol. 1994; 88:754–759. PMID:
7819100.

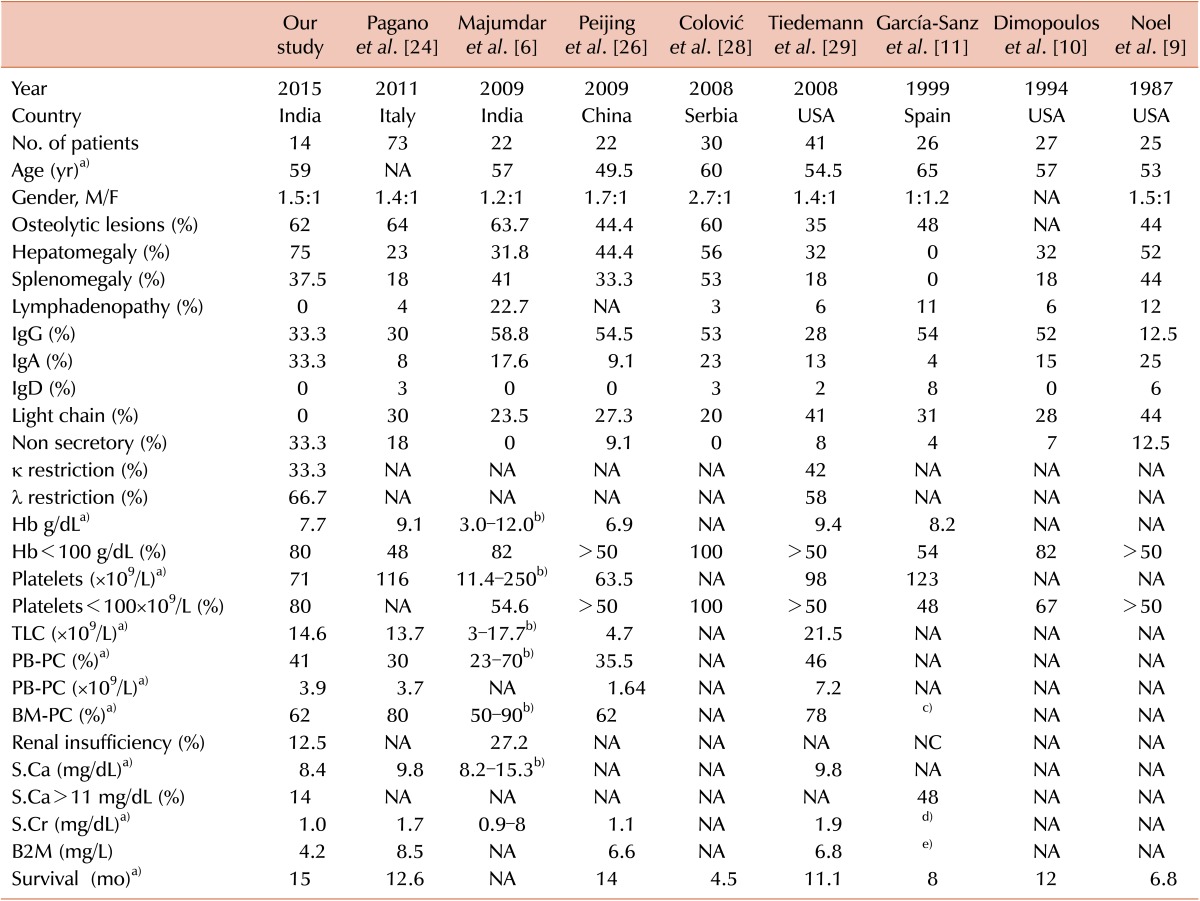
11. García-Sanz R, Orfão A, Gonzalez M, et al. Primary plasma cell leukemia: clinical, immunophenotypic, DNA ploidy, and cytogenetic characteristics. Blood. 1999; 93:1032–1037. PMID:
9920853.
12. Saccaro S, Fonseca R, Veillon DM, et al. Primary plasma cell leukemia: report of 17 new cases treated with autologous or allogeneic stem-cell transplantation and review of the literature. Am J Hematol. 2005; 78:288–294. PMID:
15795922.

13. Musto P, Rossini F, Gay F, et al. Efficacy and safety of bortezomib in patients with plasma cell leukemia. Cancer. 2007; 109:2285–2290. PMID:
17469169.

14. Albarracin F, Fonseca R. Plasma cell leukemia. Blood Rev. 2011; 25:107–112. PMID:
21295388.

15. Gogia A, Raina V, Gupta R. Massive ascites as an presenting feature of plasma cell leukemia. Indian J Cancer. 2013; 50:301.

16. Tembhare PR, Subramanian PG, Sehgal K, et al. Immunophenotypic profile of plasma cell leukemia: a retrospective study in a reference cancer center in India and review of literature. Indian J Pathol Microbiol. 2011; 54:294–298. PMID:
21623077.

17. Naseem S, Kaur S, Gupta R, Kashyap R, Nityanand S. Plasma cell leukemia: case series from a tertiary center with review of literature. Indian J Hematol Blood Transfus. 2012; 28:10–14. PMID:
23449039.

18. Kar R, Priyadarshini SG, Niraimathi M, Basu D, Badhe BA. Clinico-pathological spectrum of primary plasma cell leukemia diagnosed at a tertiary care centre in South India over 5 year period. Indian J Hematol Blood Transfus. 2012; 28:170–174. PMID:
23997454.
19. Majhi U, Murhekar K, Sundersingh S, Rajalekshmi KR. Primary plasma cell leukaemia with unusual presentations: a case series. Indian J Hematol Blood Transfus. 2014; 30(Suppl 1):390–393. PMID:
25332628.

20. Agarwal AD, Brahmbhatt BS, Parikh BP, Shah MJ. Plasma cell leukemia: a retrospective study of 10 cases. Indian J Cancer. 2014; 51:18–19. PMID:
24947090.

21. Rajeswari G, Paul TR, Uppin MS, et al. Plasma cell leukemia: A case series from South India with emphasis on rarer variants. Indian J Med Paediatr Oncol. 2014; 35:211–214. PMID:
25336792.

22. Durie BG, Harousseau JL, Miguel JS, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006; 20:1467–1473. PMID:
16855634.

23. Gluzinski A, Reichenstein M. Myeloma und leucaemia lymphatica plasmocellularis. Wien Klin Wochenschr. 1906; 12:336–339.
24. Pagano L, Valentini CG, De Stefano V, et al. Primary plasma cell leukemia: a retrospective multicenter study of 73 patients. Ann Oncol. 2011; 22:1628–1635. PMID:
21252060.

25. Royer B, Magrangeas F, Lioure B, et al. First large prospective study for patients with primary plasma cell leukemia: Bortezomib-doxorubicine-dexamethasone/bortezomib-cyclophosphamidedexamethasone regimens as induction before stem cell transplantation followed by consolidation with lenalidomide-bortezomib-dex or allograft. (a study of the IFM group) Blood (ASH Annual Meeting Abstracts). 2013; 122(Suppl):761.
26. Peijing Q, Yan X, Yafei W, et al. A retrospective analysis of thirty-one cases of plasma cell leukemia from a single center in China. Acta Haematol. 2009; 121:47–51. PMID:
19339770.

27. van de Donk NW, Lokhorst HM, Anderson KC, Richardson PG. How I treat plasma cell leukemia. Blood. 2012; 120:2376–2389. PMID:
22837533.

28. Colović M, Janković G, Suvajdzić N, Milić N, Dordević V, Janković S. Thirty patients with primary plasma cell leukemia: a single center experience. Med Oncol. 2008; 25:154–160. PMID:
18488157.

29. Tiedemann RE, Gonzalez-Paz N, Kyle RA, et al. Genetic aberrations and survival in plasma cell leukemia. Leukemia. 2008; 22:1044–1052. PMID:
18216867.

30. Toma VA, Retief FP, Potgieter GM, Anderson JD. Plasma cell leukaemia. Diagnostic problems in our experience with 11 cases. Acta Haematol. 1980; 63:136–145. PMID:
6769279.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download