Introduction
Copper is an essential element for all living organisms, because it has key activities in the metabolic enzymes such as cytochrome oxidase and superoxide dismutase, and in the proteins essential for iron homeostasis such as ceruloplasmin and hephaestin. In humans, the daily copper requirement is low, therefore copper deficiency is not so frequently a condition. However, we should be aware that copper deficiency can cause hematological abnormalities and sometimes it may masquerade as a myelodysplastic syndrome (MDS).
Copper deficiency is mainly associated with the conditions, such as gastric and bariatric surgery, parenteral hyperalimentation, enteroparthies with loss of proteins, hypoproteinemic status such as celiac disease, complications due to therapy with high doses of zinc and penicillamine, and chronic use of proton pump inhibitors. Copper deficiency can be associated with hyperzinchemia; in some cases, this condition could be a consequence of possible use of zinc based denture adhesives creams. The copper deficiency may also be the result of an inherited disorder, such as the Menkes disease.
Go to : 
Copper deficiency and cytopenia
The most common hematological abnormailities in copper deficiency are anemia and neutropenia [1]. The pathogenesis of anemia in copper deficiency is complex and multifactorial. Copper and iron interact through the ceruloplasmin, a copper-dependent oxidase, which assists in iron transport in the plasma in association with transferrin by oxidation of Fe2+ into Fe3+ [2] (Fig. 1). The hephaestin, a transmembrane copper-containing ferroxidase, having 50% homology to ceruloplasmin, works as a facilitator for iron export from enterocytes into blood circulation.
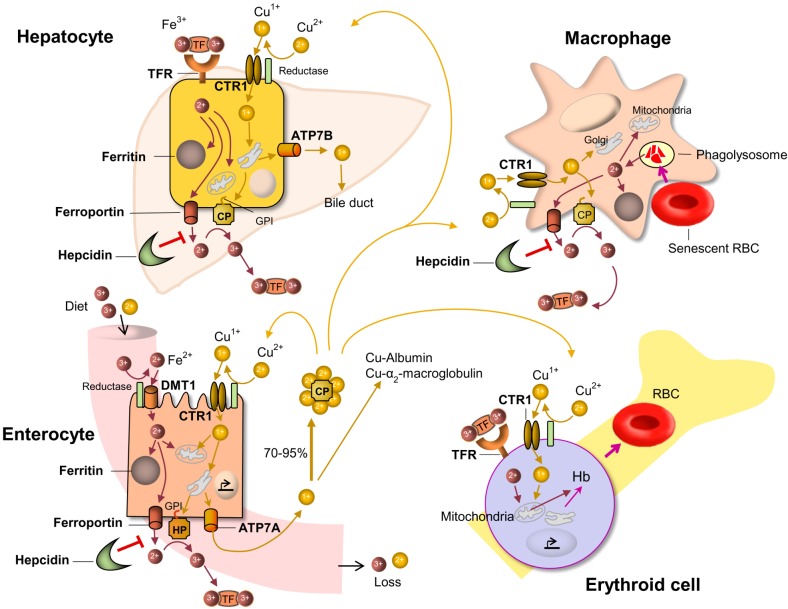 | Fig. 1The iron and copper transport pathways and their interactions. Into the enterocytes, dietary iron and copper are absorbed by DMT1 and CTR1, repectively, after reduction. Iron and copper are exported from enterocyte by ferroportin and ATP7A, respectively. Copper deficiency decreases the quantity of HP which is required for efficient enterocyte iron efflux and loading onto TF. CP carries 70–95% of the total copper in plasma. Hepatocytes can take up Fe-TF by TFR. Copper taken up by hepatocyte CTR1 is delivered to ATP7B and CP. CP facilitates iron loading onto TF by oxidation (Fe2+→Fe3+). Hepatocytes control iron metabolism by producing the hepcidin which inhibits iron export. Fe-TF is transported to the marrow and utilized for the erythropoietic activity. Iron taken up by erythrocyte, the major iron consumer, is transferred to the mitochondria together with copper, and used for heme systhesis. Copper deficiency can impair the uptake of iron by mitochondria and cause decrease of heme synthesis. Macrophage phagocytoses senescent RBC and the irons are utilized or stored in ferritin.Abbreviations: ATP7A, ATPase Cu2+ transporting alpha polypeptide; ATP7B, ATPase Cu2+ transporting beta polypeptide; CP, ceruloplasmin; CTR1, copper transporter 1; DMT1, divalent metal transporter 1; Hb, hemoglobin; HP, hephaestin; GPI, glycosylphosphatidylinositol; RBC, red blood cell; TF, transferrin; TFR, transferrin receptor.
|
Incorporated into ceruloplasmin, the copper is essential to mobilize the iron from the liver and transport to the bone marrow where it is utilized for erythropoiesis. In case of copper defiency, iron accumulates in the liver and iron availability is decreased in circulation and bone marrow, consequently copper deficiency causes an ineffective erythropoiesis [1].
Patients with copper deficiency manifest an evident insufficiency of hematopoiesis characterized by anemia and leukopenia, and less frequently, thrombocytopenia [3]. In anemia caused by copper deficiency, the erythrocyte mean corpuscular volume (MCV) may be normal, low or increased, resulting in normocytic, microcytic or macrocytic anemia. Behind the most common anemia caused by iron deficiency or vitamin B12 and/or folate deficiencies, it is possible in some cases that complex multifactorial conditions including copper deficiency can be hidden. These conditions may show complex erythrocyte morphological features [4].
The mechanism by which neutropenia develops in copper deficiency is not clear. Probably it may be caused by decreased survival of circulating neutrophils,or by inhibition of differentiation and self-renewal of CD34(+) hematopoietic progenitor cells [5]. Low serum copper levels directly support the diagnosis of copper deficiency. Although ceruloplasmin binds 70–95% of copper and is responsible for its transport, its plasma level can not be specific for copper deficiency, because it is also a reactive protein of acute phase.
Go to : 
Copper deficiency and myelodysplasia
Copper deficiency, in addition to causing cytopenia, can also generate dysplastic hematopoietic features, and sometimes it mimics MDS. However, of course, the karyotype does not show cytogenetic abnormalities. In bone marrow, erythroblasts and granulocytic precursors manifest intracytoplasmatic vacuoles [6]. As part of erythroblastic dysplasia, in copper deficiency, ringed sideroblasts can also be detected, and in this case, copper supplement can correct the anemia in contrary to cases of clonal refractory anemia with ringed sideroblasts [7]. The presence of intracytoplasmatic iron granules in plasma cells is another possible morphological appearance that can be recognized in bone marrow [8]. In addition to erythroblasts and myeloid precursors dysplasia, hematogone hyperplasia can be detected by flow cytometry in copper deficiency [9]. In cytopenic patients, a low or absent hematogone number can represent another important requirement to distinguish between MDS and copper deficiency-related dysplasia. Before making a diagnosis of MDS, it is suggested that copper deficiency should be ruled out. Although 10% of dysplastic cells in any hematopoietic cell line is the threshold for the diagnosis of MDS, it should be noted that an excess of 10% may be also found in some normal subjects and often in non-neoplastic cytopenia [10].
Go to : 
Conclusion
It is well known that copper deficiency can induce hematological abnormalities. In copper deficiency, commonly observed abnormalities in bone marrow include vacuoles in myeloid precursors, iron-containing plasma cells, a decrease in granulocyte precursors and ring sideroblasts. Sometimes these features may lead to misdiagnosis as MDS. In patients with cytopenia and/or low-grade MDS, copper dosage can be appropriate. However, it should be emphasized that copper deficiency is treatable by copper therapy. Copper supplement per os, or parenteral copper chloride in case of enteropathies, leads to significant improvement.
Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download