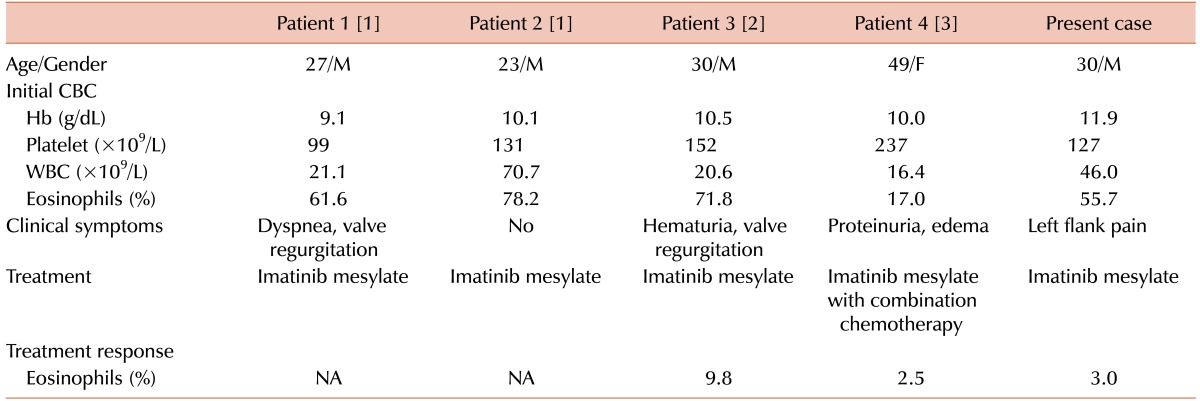
TO THE EDITOR: We read an interesting paper by Shin et al. [1] in a recent issue of Blood Research, titled "Chronic eosinophilic leukemia (CEL) with a FIP1L1-PDGFRA rearrangement: Two case reports and a review of Korean cases". To our knowledge, CEL with FIP1L1-PDGFRA rearrangement is very rare in Korea, and only 4 cases have been reported to date [123] (Table 1). In this letter, we report an additional rare case of CEL with FIP1L1-PDGFRA rearrangement and briefly discuss the possibilities of ethnical differences of this rare gene rearrangement, specifically in the Korean population.
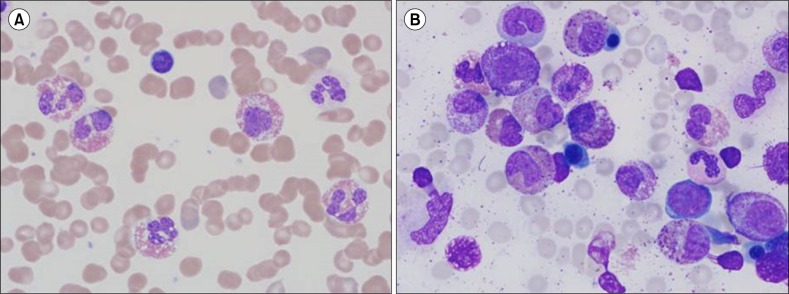
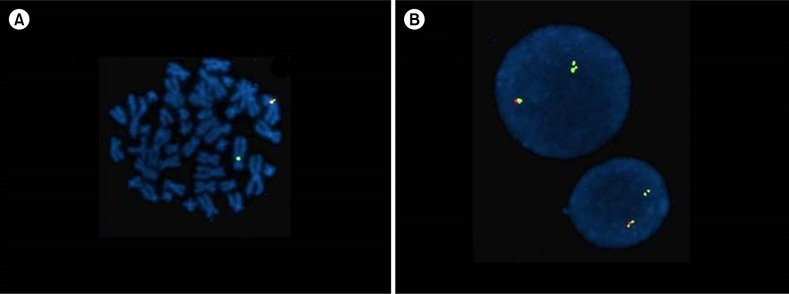
A 30-year-old Korean man was admitted to our hospital due to left flank pain associated with ureter stone. Initial complete blood count (CBC) showed a hemoglobin (Hb) level of 11.9 g/dL (reference range, 13–17 g/dL) and a platelet count of 127,000/µL (reference range, 150,000–350,000/µL) with a white blood cell (WBC) count of 46,030/µL (reference range, 4,000–10,000/µL): 30.6% neutrophils, 7% lymphocytes, 3.9% monocytes, 55.7% eosinophils, and 1% basophils. Peripheral blood (PB) smear also showed increased number of eosinophils (Fig. 1A). Blood urine nitrogen (BUN) and creatinine levels were within reference ranges. Causes of eosinophilia due to allergy and parasite infections were excluded from further studies. Bone marrow examination was performed, showing hypercellular marrow with eosinophilic precursors, including mature eosinophils, being counting up to 36.2% (Fig. 1B). The chromosome study showed a normal karyotype; however, the FIP1L1-PDGFRA rearrangement was detected by fluorescence in situ hybridization (FISH) analysis (Fig. 2). Diagnosis of CEL with FIP1L1-PDGFRA rearrangement was made. Our patient was treated with daily administration of imatinib mesylate (100 mg) and followed up with CBC. After 1 year, the follow up CBC showed normalized leukocyte count of 8,170/µL with 3% eosinophils. Furthermore, eosinophil precursors were within reference range of bone marrow aspiration. FISH analysis using a FIP1L1-PDGFRA probe showed normal signal patterns (without deletion signals).
Within the distinct disease entity of myeloid and lymphoid neoplasms with eosinophilia and abnormalities of PDGFRA, PDGFRB, or FGFR1, FIP1L1-PDGFRA is the most common of three genetic abnormalities associated with CEL, although its incidence among the general population is fairly low. It also has a male predilection with male to female ratio of 17:1, and a median age of onset late in fifth decade of life [4].
Representative examples of ethnic differences among genetic abnormalities of hematologic malignancy include a higher incidence of acute promyelocytic leukemia in Chinese populations and the comparably low incidence of chronic lymphocytic leukemia among Asian ethnicity. Interestingly, most CEL cases with FIP1L1-PDGFRA rearrangement in the Korean population were male patients in their early third and fourth decades of life, with the exception of a female patient in her forties, suggestive of an earlier onset among the Korean population. However, this conclusion is premature because there have only been five Korean cases reported, including our case. Nation-wide, multicenter research should properly assess the incidence and clinical characteristics, such as early onset and patients' responses to therapy.
Acknowledgments
This work was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2014R1A1A1002797).
References
1. Shin SY, Jung CW, Choi DC, Lee BJ, Kim HJ, Kim SH. Chronic eosinophilic leukemia with a FIP1L1-PDGFRA rearrangement: Two case reports and a review of Korean cases. Blood Res. 2015; 50:58–61. PMID: 25830134.
2. Jeung YJ, Lee JY, Lee GY, Oh MJ, Lee BJ, Choi DC. A case of idiopathic hypereosinophilic syndrome presenting with nocturia and dyspnea. Korean J Asthma Allergy Clin Immunol. 2010; 30:241–245.
3. Kim DW, Shin MG, Yun HK, et al. Incidence and causes of hypereosinophilia (corrected) in the patients of a university hospital. Korean J Lab Med. 2009; 29:185–193. PMID: 19571614.
4. Swerdlow SH, Campo E, Harris NL, editors. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon, France: IARC Press;2008.
Fig. 1
Morphology in a patient of chronic eosinophilic leukemia with a FIP1L1-PDGFRA rearrangement. (A) The number of eosinophils increased (55.7%) in the peripheral blood (PB) smear (Wright-Giemsa, ×1,000). (B) The number of eosinophilic precursors and eosinophils was markedly increased, and equaled up to 36.2% in the bone marrow (BM) aspiration (Wright-Giemsa, ×1,000). Dysplastic eosinophils, including sparse granulation, nuclear hypersegmentation or hyposegmentation are shown in PB smear and BM aspiration (A, B).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download