Abstract
Background
This study aimed to assess the treatment outcomes of ifosphamide, mesna, etoposide, and prednisolone (IMEP) combination regimen as a front-line chemotherapy in patients with peripheral T-cell lymphomas (PTCLs).
Methods
Clinical data of 38 newly diagnosed PTCLs patients who underwent IMEP at Busan Paik Hospital from January 2002 to December 2013 were retrospectively analyzed.
Results
The overall response rate was 68.5%, with 21 (55.3%) complete response/complete response unconfirmed and 6 (15.8%) partial response (PR). The median follow-up duration was 25.5 months (range, 0.2-87.3). The median overall survival was not reached and 2-year survival rate was 67%. The median progression free survival was 23 months. The most frequently reported adverse effects higher than grade 3 were hematologic toxicities including neutropenia (68.4%), thrombocytopenia (42.1%). There was no treatment-related mortality.
Peripheral T-cell lymphomas (PTCLs) are a type of non-Hodgkin lymphoma (NHL) derived from mature T cells. They account for approximately 10–15% of all NHLs in the United States and Europe. The incidence of these lymphomas is higher in Asia than in Western countries, and is reported to be up to 20% [1]. In the Korean population, the incidence of PTCLs is 19.5% according to a recent study involving approximately 3,800 NHL patients [2].
The PTCLs comprise a heterogeneous group of lymphomas, and the prognosis of the disease differs according to the subtypes. Peripheral T-cell lymphoma not otherwise specified (PTCL-NOS), angioimmunoblastic T-cell lymphoma (AITL), NK/T-cell lymphoma (NKTCL), adult T-cell leukemia/lymphoma (ATLL), and anaplastic large cell lymphoma (ALCL) are the most common distinct pathologic subtypes and comprise most PTCLs. The other types include primary cutaneous ALCL, hepatosplenic T-cell lymphoma, and subcutaneous panniculitis-like T cell lymphoma, which account for less than 2% of all PTCLs. The 5-year survival rate of patients who are diagnosed with PTCL-NOS, AITL, or NKTCL is 32%; ATLL, 14%; and anaplastic lymphoma kinase (ALK)-negative ALCL, 49%, whereas, the 5-year survival rate of patients with ALK-positive ALCL is 70%. Except for ALK-positive ALCL, PTCLs show a poor prognosis compared with aggressive B-cell lymphomas [3].
The optimal therapy of PTCLs has not been established. National Comprehensive Cancer Network guidelines recommend clinical trials as a preferred option for treatment of PTCLs. Anthracycline-based regimens, including CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone), have been traditionally been used for treatment of PTCLs, similar to diffuse large B-cell lymphoma (DLBCL). However, the complete remission rate and 5-year overall survival after a CHOP regimen in PTCL patients is about 10% lower than that of DLBCL patients [4]. Moreover, anthracycline-containing regimens did not show a clinical benefit over a non–anthracycline-containing regimen in patients with PTCL or NKTCL, except for ALK-positive ALCL [3]. Consequently, new combination therapies without anthracycline are being explored in many studies.
Ifosfamide, mesna, methotrexate, etoposide, and prednisolone (IMEP) combination chemotherapy was initially developed as a salvage therapy for recurrent lymphoma. In 1982, Cabanillas et al. [5] reported the clinical outcome of the IMVP-16 regimen consisting of ifosfamide, methotrexate, and VP-16 in patients with recurrent lymphoma. In that study, the IMVP-16 regimen showed a complete remission rate of 37% and a median survival of 15 months. Other studies also demonstrated that the IMVP-16 regimen was effective as a salvage therapy for relapsed/refractory NHL [67]. There has been lack of data about IMEP as a front-line therapy in PTCLs. In the present study, we retrospectively analyzed the treatment outcome of IMEP combination chemotherapy as a front-line therapy for patients with PTCLs.
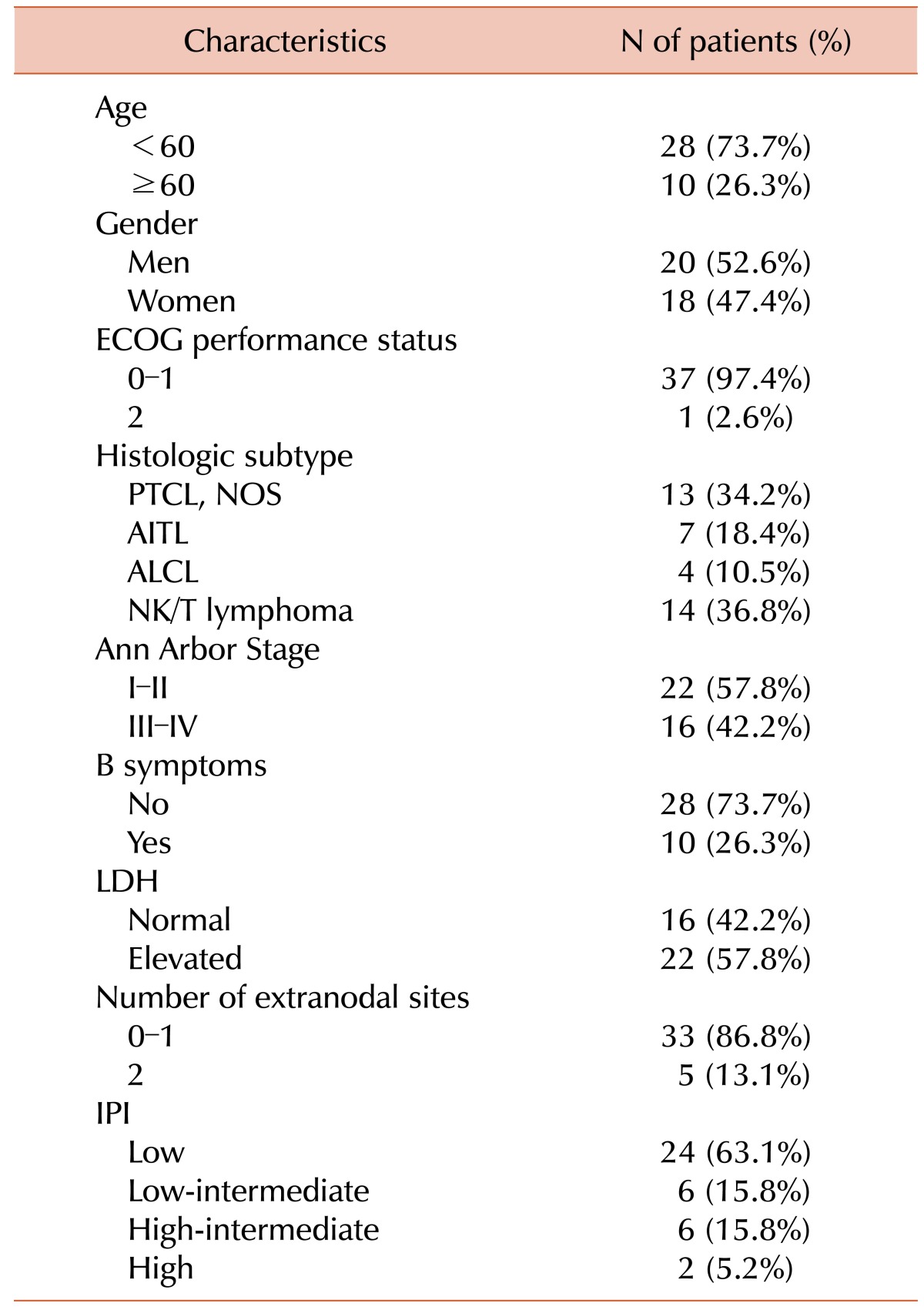
From January 2002 to December 2013, a total of 38 patients who were newly diagnosed with PTCL and who received IMEP as front-line chemotherapy at Busan Paik Hospital were enrolled in the study. Patients were included if they were pathologically confirmed as having PTCL according to the World Health Organization (WHO) classification and had received no prior chemotherapy. Cutaneous T cell lymphoma and lymphoblastic lymphoma/leukemia were excluded. The clinical data of patients were retrospectively analyzed, and the final date of data collection was December 31, 2014. Clinical characteristics, such as age, gender, B symptoms, serum lactate dehydrogenase (LDH) levels, number of extranodal sites, Ann-Arbor stage, International Prognostic Index (IPI) score, pathologic subtype, Eastern Cooperative Oncology Group (ECOG) performance status, and survival data were analyzed. The Institutional Review Board of the Busan Paik Hospital has approved this retrospective study (2015-01-0005).
All patients received IMEP chemotherapy consisting of ifosfamide (1.5 g/m2) intravenously (IV) on days 1–3 with adequate hydration and mesna to prevent hemorrhagic cystitis; methotrexate (30 mg/m2 IV) on days 3 and 10; etoposide (100 mg/m2 IV) on days 1–3; and prednisolone (60 mg/m2 per os) on days 1–5. Treatment was repeated every 3 weeks. After 3 courses of therapy, patients were re-evaluated via physical examination and computed tomography. Responding patients according to International Working Group criteria [8] were allowed to continue the next cycle and received a total of 6 cycles of IMEP. In the case of disease progression during IMEP therapy, IMEP was discontinued and salvage chemotherapy was administered. Adverse events were assessed according to the National Cancer Institute's Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 4.0. In patients whose neutrophil count was <1.5×109/L or platelet count was <100×109/L before a scheduled cycle, the cycle was delayed for 1 week. If febrile neutropenia or grade 4 neutropenia occurred, the dose of ifosfamide, methotrexate, and etoposide was reduced by 25%.
Overall survival (OS) was calculated from the date of initiation of IMEP chemotherapy to the date of death from any cause or the last follow-up visit. Progression-free survival (PFS) was calculated from the date of initiation of IMEP chemotherapy to date of disease progression, death, or the last follow-up visit. The Kaplan-Meier method was used to analyze the survival data. Comparisons of survival were made using the log-rank test. Univariate analyses of independent factors for survival were performed using the Cox proportional hazard model. P-values<0.05 were considered statistically significant. Statistical analysis was performed using SPSS version 21.0 (IBM corporation, NY, US).
Patient characteristics are shown in Table 1. The median age was 50 years (range, 17–76); 10 (26.4%) were elderly (≥60 yr). There were 20 (52.6%) men and 18 (47.4%) women. Almost all patients had a good performance status of ECOG 0–1. The most common histologic subtypes were NKTCL (36.8%) and PTCL-NOS (34.2%). B symptoms were observed in 10 (26.3%) patients. Sixteen (42.2%) patients had Ann Arbor stage III or IV tumors at diagnosis, and 8 (21%) patients were at high-intermediate or high-risk according to their IPI scores.
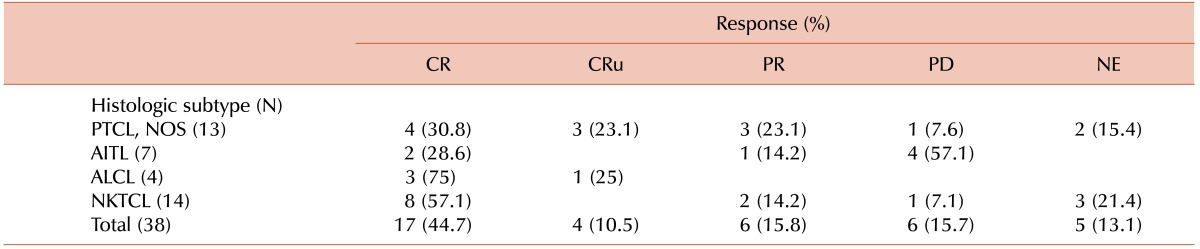
Out of 38 patients, 17 (44.7%) had a complete response (CR), 4 (10.5%) had a complete response, unconfirmed (CRu), 6 (15.8%) had a partial response (PR), and 6 (15.8%) had progressive disease (PD). Response could not be evaluated in 5 patients. Of the 5 patients who were not evaluated for a response, 3 patients refused further evaluation and treatment due to deterioration of their general condition, 1 patient transferred to another hospital, and 1 patient died of pneumonia before response evaluation. The overall response rate was 71%. One hundred percent (4/4) and 57.1% (8/14) of patients with ALCL and NKTCL had a CR/CRu, respectively. The responses according to histologic subtype are shown in Table 2.
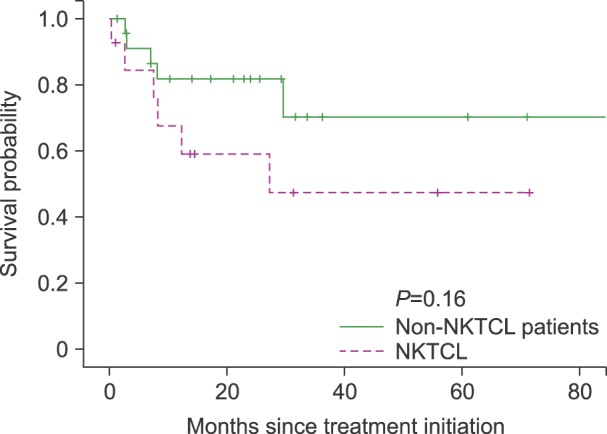
A total of 11 patients died, 9 from disease progression, 1 from pneumonia 1 week after the 1st cycle of IMEP combination chemotherapy, and 1 of unknown causes. The median follow-up duration was 25.5 months (range, 0.2–87.3). The median OS was not reached, and the 2-year survival rate was 67%. The median PFS was 23 months (Fig. 1). There were no significant differences in survival between patients with NKTCL histology versus non-NKTCL histology (P=0.16, Fig. 2).
Of 17 complete responders, 11 patients were still alive and maintaining a CR. Nine of these patients visited the hospital regularly for the purpose of surveillance, 2 underwent up-front autologous stem cell transplantation. The median duration of a CR was 15.5 months (range, 0.69-34.2).
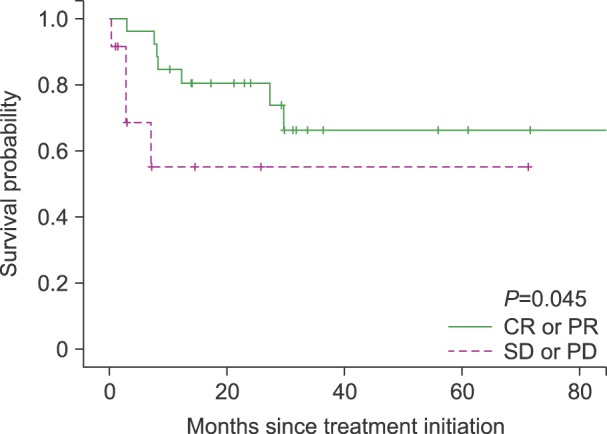
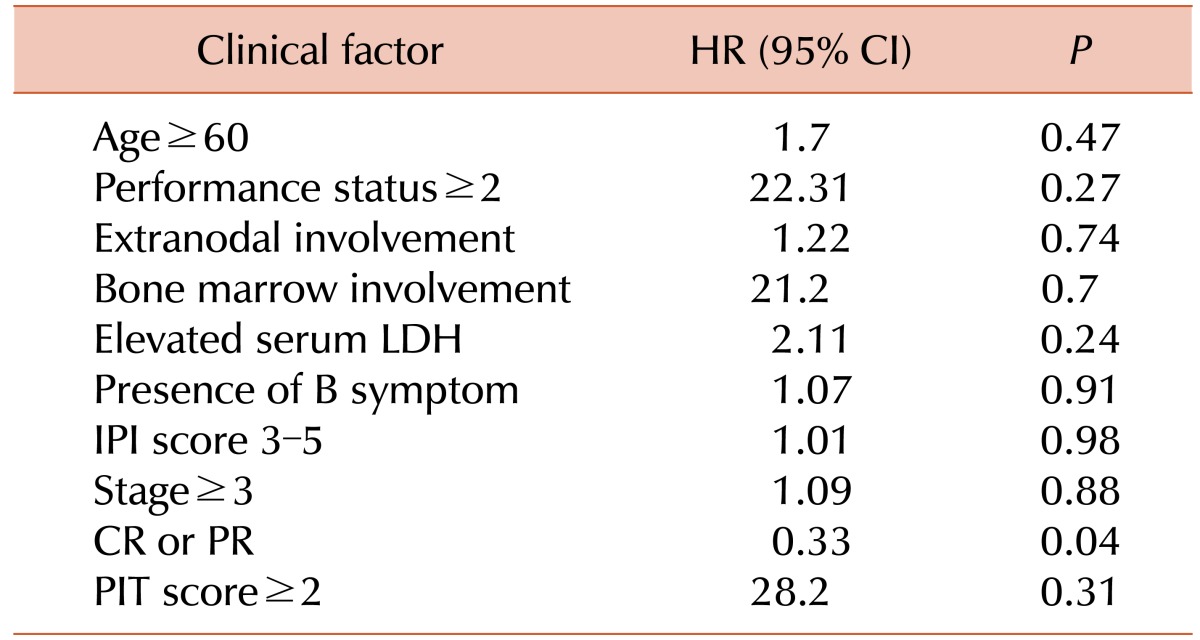
Univariate analysis was performed to identify prognostic factors (Table 3). Response to treatment (CR/CRu or PR) was the only significant prognostic factor for survival (P=0.045, Fig. 3). There was a weak tendency toward better survival outcome in patients who achieved CR/CRu than patients who did not, however, it did not show statistical significance (P=0.54). Age, performance status, extranodal involvement, bone marrow involvement, serum LDH level, presence of B symptoms, IPI score, PIT score, and stage did not show a significant association with survival.
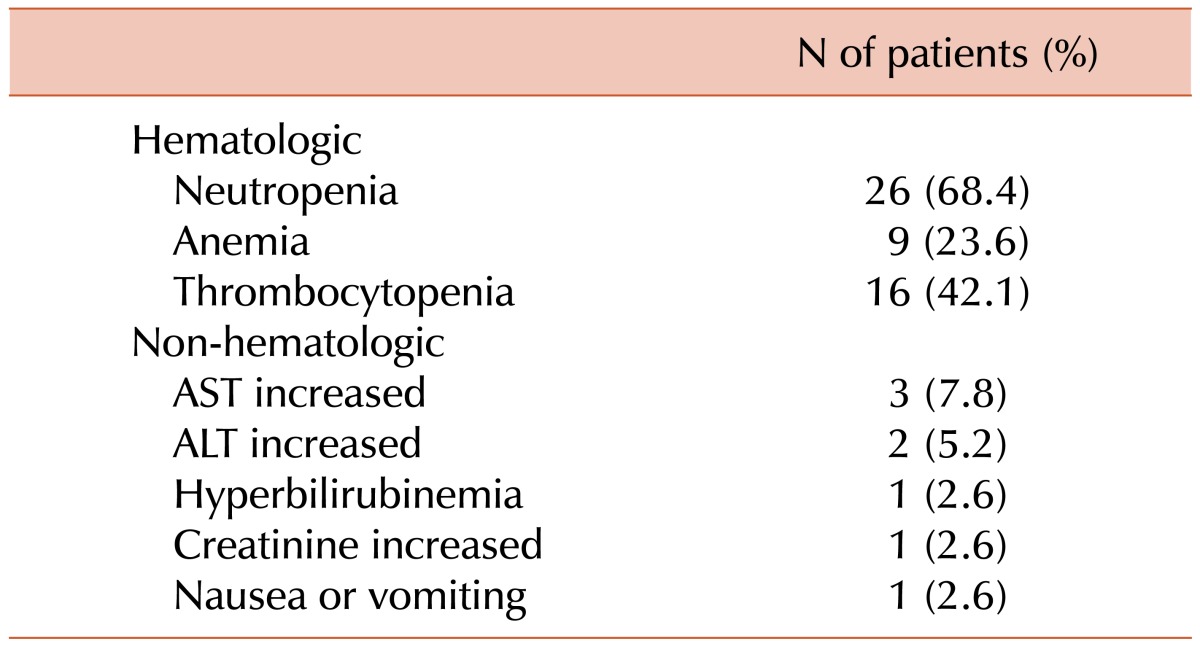
Toxicity profiles are shown in Table 4. A total of 178 of the 228 planned cycles (78%) were delivered. The median number of cycles administered was 6 (range, 1–6). Twenty-one patients completed a total of 6 cycles. The most frequently reported adverse effects higher than grade 3 were hematologic toxicities, including neutropenia (68.4%) and thrombocytopenia (42.1%). Febrile neutropenia occurred in 5 (13.1%) patients. All patients with febrile neutropenia recovered after treatment with granulocyte colony stimulating factor and antibiotics. Treatment-related mortality was observed in 1 patient, a 45-year-old woman with NKTCL whose IPI score was 3 and whose Ann-Arbor stage was 4. She died of pneumonia and sepsis 1 week after the 1st cycle of IMEP combination chemotherapy. Grade 3–4 non-hematologic toxicities affected fewer than 10% of patients.
PTCLs have traditionally been managed with anthracycline-based regimens, but they have shown poor outcomes compared with B-cell lymphomas, with a low complete response (CR) rate and poor survival in some studies (Table 5). In a recent phase III study that compared the efficacy of CHOP-21 versus VIP-rABVD (etoposide, ifosfamide, cisplatin-reinforced-doxorubicin, bleomycin, vinblastine, and dacarbazine) in 88 newly diagnosed PTCL patients, the CHOP-21 arm showed a CR rate 33.3%, an overall response rate of 66.6%, and a median survival of 42 months [9]. In the present study, we retrospectively assessed the efficacy of the IMEP regimen as a front-line chemotherapy, and found a CR/CRu rate of 55.3% and a 2-year OS of 67%. It seems to be as effective as the traditionally used anthracycline-based regimens.
Only a few studies have evaluated the efficacy of the IMEP regimen as a front-line therapy in PTCLs. Some authors reported that the IMEP regimen showed a variable CR rate (27–66%) and response rate (73%) in newly diagnosed NKTCL patients as a front-line therapy [101112]. It is difficult to directly compare the outcome of our study with these studies on NKTCL patients, but the CR/CRu rate (55.3%) and overall response rate (71%) of our study does not seem to be inferior.
A phase II study conducted in 44 stage I/II NKTCL patients showed a 27% CR rate after IMEP alone and a 67% CR rate after IMEP followed by radiotherapy [12]. They suggest that IMEP followed by radiotherapy may be more efficacious in patients who cannot achieve a CR after chemotherapy alone. In our study, out of 14 NKTCL patients, 11 were stage I/II and achieved a CR rate of 63% after IMEP chemotherapy alone. There were 2 patients with a PR, 1 who underwent up-front autologous stem cell transplantation without radiotherapy and 1 who was lost to follow-up after achieving a PR. We could not access the efficacy of additional radiotherapy, because few patients received radiotherapy in our study. Moreover, the lack of baseline clinical data related to response, such as pretreatment Epstein-Barr virus, made it hard to compare studies directly. At least, the outcome of our study seems not to be inferior to previous studies.
In a retrospective analysis of 32 patients with relapsed or refractory PTCLs patients who were treated with IMEP followed by autologous stem cell transplantation, the IMEP regimen seemed to be ineffective in non-NKTCL cases [13]. Our study showed a similar trend, in that patients with NKTCL had a higher CR/CRu rate (57.1%) than did PTCL-NOS (53.9%) or AITL (28.6%).
Age>60 years, poor performance status, 2 extranodal sites, and high IPI are known prognostic factors that predict worse survival [14]. We could not find any significant prognostic factors in our study, except for response to therapy. Patients who achieve CR/CRu or PR had a longer survival than who did not. The number of patients in our study is too small to produce confirmative results, and further investigations are required.
Compared with anthracycline-based regimens for NHL [1516], IMEP was generally tolerable. Thrombocytopenia (42.1%) seems to be more frequent than in anthracycline-based regimen; however, all patients fully recovered from thrombocytopenia without any adverse events related to bleeding. Considering that 26% of patients were older than 60 years in our study, IMEP regimen is also tolerable in elderly patients.
In conclusion, the IMEP regimen seems to be effective and safe as a front-line chemotherapy in patients with PTCLs. Future trials studying effective chemotherapy for PTCLs are needed.
References
1. Armitage JO. The aggressive peripheral T-cell lymphomas: 2013. Am J Hematol. 2013; 88:910–918. PMID: 24078271.

2. Kim JM, Ko YH, Lee SS, et al. WHO classification of malignant lymphomas in Korea: Report of the third nationwide study. Korean J Pathol. 2011; 45:254–260.

3. Vose J, Armitage J, Weisenburger D. International T-Cell Lymphoma Project. International peripheral T-cell and natural killer/T-cell lymphoma study: Pathology findings and clinical outcomes. J Clin Oncol. 2008; 26:4124–4130. PMID: 18626005.
4. Gisselbrecht C, Gaulard P, Lepage E, et al. Groupe d'etudes des lymphomes de l'adulte (GELA). Prognostic significance of T-cell phenotype in aggressive non-hodgkin's lymphomas. Blood. 1998; 92:76–82. PMID: 9639502.
5. Cabanillas F, Hagemeister FB, Bodey GP, Freireich EJ. IMVP-16: An effective regimen for patients with lymphoma who have relapsed after initial combination chemotherapy. Blood. 1982; 60:693–697. PMID: 7104493.

6. Huijgens PC, Ossenkoppele GJ, Van Der Lelie J, Thomas LL, Wijngaarden MJ, Slaper CM. IMVP-16 followed by high dose chemotherapy and autologous bone marrow transplantation as salvage treatment for malignant lymphoma. Hematol Oncol. 1991; 9:245–251. PMID: 1743627.

7. Aurer I, Duraković N, Radman I, et al. Combination of ifosfamide, methotrexate, and etoposide (IMVP) as a salvage therapy for relapsed and refractory aggressive non-Hodgkin lymphoma: Retrospective study. Croat Med J. 2002; 43:550–554. PMID: 12402394.
8. Cheson BD, Horning SJ, Coiffier B, et al. NCI Sponsored International Working Group. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. J Clin Oncol. 1999; 17:1244. PMID: 10561185.

9. Simon A, Peoch M, Casassus P, et al. Upfront VIP-reinforced-ABVD (VIP-rABVD) is not superior to CHOP/21 in newly diagnosed peripheral T cell lymphoma. Results of the randomized phase III trial GOELAMS-LTP95. Br J Haematol. 2010; 151:159–166. PMID: 20738307.

10. Lee KW, Yun T, Kim DW, et al. First-line ifosfamide, methotrexate, etoposide and prednisolone chemotherapy +/- radiotherapy is active in stage I/II extranodal NK/T-cell lymphoma. Leuk Lymphoma. 2006; 47:1274–1282. PMID: 16923557.

11. Lee KW, Kim DW, Im SA, et al. Efficacy of ifosfamide, methotrexate, etoposide and prednisone (IMEP) chemotherapy in extranodal NK/T-cell lymphoma, nasal type. J Clin Oncol (ASCO Annual Meeting Abstracts). 2004; 22(Suppl):abst 6685.

12. Kim TM, Kim DW, Kang YK, et al. A phase II study of ifosfamide, methotrexate, etoposide, and prednisolone for previously untreated stage I/II extranodal natural killer/T-cell lymphoma, nasal type: A multicenter trial of the Korean Cancer Study Group. Oncologist. 2014; 19:1129–1130. PMID: 25280488.

13. Park BB, Kim WS, Lee J, et al. IMVP-16/Pd followed by high-dose chemotherapy and autologous stem cell transplantation as a salvage therapy for refractory or relapsed peripheral T-cell lymphomas. Leuk Lymphoma. 2005; 46:1743–1748. PMID: 16263576.

14. Kim M, Kim TM, Kim KH, et al. Ifosfamide, methotrexate, etoposide, and prednisolone (IMEP) plus L-asparaginase as a first-line therapy improves outcomes in stage III/IV NK/T cell-lymphoma, nasal type (NTCL). Ann Hematol. 2015; 94:437–444. PMID: 25300500.

15. Ohmachi K, Tobinai K, Kobayashi Y, et al. Phase III trial of CHOP-21 versus CHOP-14 for aggressive non-Hodgkin's lymphoma: Final results of the Japan Clinical Oncology Group Study, JCOG 9809. Ann Oncol. 2011; 22:1382–1391. PMID: 21196441.

16. Pfreundschuh M, Trumper L, Kloess M, et al. Two-weekly or 3-weekly CHOP chemotherapy with or without etoposide for the treatment of young patients with good-prognosis (normal LDH) aggressive lymphomas: Results of the NHL-B1 trial of the DSHNHL. Blood. 2004; 104:626–633. PMID: 14982884.

17. Wöhrer S, Chott A, Drach J, et al. Chemotherapy with cyclophosphamide, doxorubicin, etoposide, vincristine and prednisone (CHOEP) is not effective in patients with enteropathy-type intestinal T-cell lymphoma. Ann Oncol. 2004; 15:1680–1683. PMID: 15520071.

18. Kim JG, Sohn SK, Chae YS, et al. CHOP plus etoposide and gemcitabine (CHOP-EG) as front-line chemotherapy for patients with peripheral T cell lymphomas. Cancer Chemother Pharmacol. 2006; 58:35–39. PMID: 16308699.

19. Sung HJ, Kim SJ, Seo HY, et al. Prospective analysis of treatment outcome and prognostic factors in patients with T-cell lymphomas treated by CEOP-B: Single institutional study. Br J Haematol. 2006; 134:45–53. PMID: 16803566.

20. Escalón MP, Liu NS, Yang Y, et al. Prognostic factors and treatment of patients with T-cell non-Hodgkin lymphoma: The M. D. Anderson Cancer Center experience. Cancer. 2005; 103:2091–2098. PMID: 15816054.
21. Morabito F, Gallamini A, Stelitano C, et al. Clinical relevance of immunophenotype in a retrospective comparative study of 297 peripheral T-cell lymphomas, unspecified, and 496 diffuse large B-cell lymphomas: Experience of the Intergruppo Italiano Linformi. Cancer. 2004; 101:1601–1608. PMID: 15378507.
22. Savage KJ, Chhanabhai M, Gascoyne RD, Connors JM. Characterization of peripheral T-cell lymphomas in a single North American institution by the WHO classification. Ann Oncol. 2004; 15:1467–1475. PMID: 15367405.

23. López-Guillermo A, Cid J, Salar A, et al. Peripheral T-cell lymphomas: Initial features, natural history, and prognostic factors in a series of 174 patients diagnosed according to the R.E.A.L. classification. Ann Oncol. 1998; 9:849–855. PMID: 9789607.

Fig. 1
Overall survival and progression free survival (A) overall survival (B) Progression-free survival.
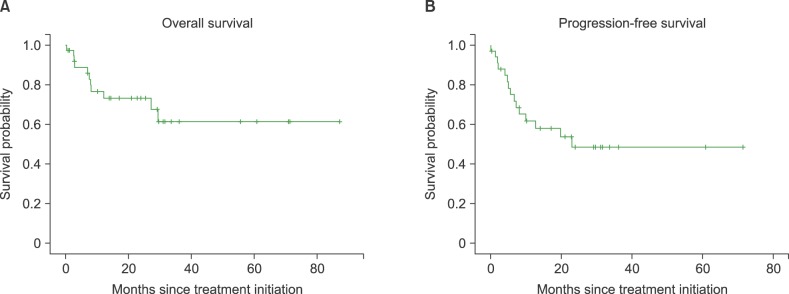
Table 5
Front-line anthracycline-based chemotherapy in PTCLs.
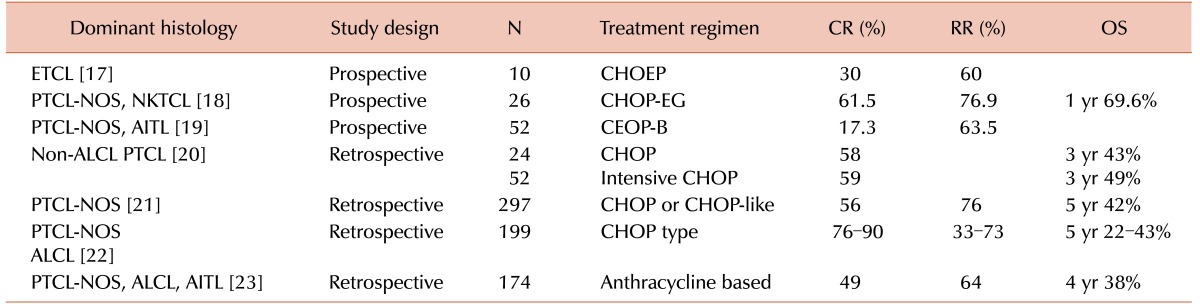
Abbreviations: CR, complete response; RR, response rate; OS, overall survival; ETCL, enteropathy-type intestinal T-cell lymphoma; PTCL-NOS, peripheral T-cell lymphoma not otherwise specified; NKTCL, NK/T-cell lymphoma; AITL, angioimmunoblastic T-cell lymphoma; ALCL, anaplastic large cell lymphoma; CHOEP, cyclophosphamide, doxorubicin, vincristine, etoposide and prednisone; CHOP-EG CHOP, plus etoposide and gemcitabine; CEOP-B, cyclophosphamide, epirubicin, vincristine, and prednisone; CHOP, cyclophosphamide, doxorubicin, vincristine and prednisone.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download