Abstract
Background
Unfractionated heparin (UFH) has unstable pharmacokinetics and requires close monitoring. The activated partial thromboplastin time (aPTT) test has been used to monitor UFH therapy for decades in Korea, but its results can be affected by numerous variables. We established an aPTT heparin therapeutic range (HTR) corresponding to therapeutic anti-Xa levels for continuous intravenous UFH administration, and used appropriate monitoring to determine if an adequate dose of UFH was applied.
Methods
A total of 134 ex vivo samples were obtained from 71 patients with a variety of thromboembolisms. All patients received intravenous UFH therapy and were enrolled from June to September 2015 at Gyeongsang National University Hospital. All laboratory protocols were in accordance with the Clinical and Laboratory Standards Institute guidelines and the College of American Pathologist requirements for aPTT HTR.
Results
An aPTT range of 87.1 sec to 128.7 sec corresponded to anti-Xa levels of 0.3 IU/mL to 0.7 IU/mL for HTR under our laboratory conditions. Based on their anti-Xa levels, blood specimen distribution were as follows: less than 0.3 IU/mL, 65.7%; 0.3–0.7 IU/mL (therapeutic range), 33.6%; and more than 0.7 IU/mL, 0.7%. No evidence of recurring thromboembolism was observed.
Go to : 
Unfractionated heparin (UFH) is the best-known anticoagulant. Although low-molecular-weight heparin has replaced a considerable portion of UFH use after its introduction into clinical practice, UFH still has many advantages, including its short half-life and reversibility by protamine sulfate. Moreover, UFH is still useful for patients with thromboembolism [123]. However, the unstable pharmacokinetics of UFH hinder its wider usage. Close monitoring and timely dose adjustments are both crucial for maintaining the therapeutic range of UFH. To monitor UFH therapy, both activated partial thrombin time (aPTT) and chromogenic anti-factor Xa (anti-Xa) assays have been used. The chromogenic anti-Xa assay is not influenced by elevated concentrations of factor VIII or fibrinogen. Moreover, this assay is not affected by any factor deficiencies with the exception of anti-thrombin deficiency. The anti-Xa assay appears to be a better method for monitoring heparin than the aPTT test because pre-analytical variables do not affect its results. In addition, the anti-Xa assay is less susceptible to other variables [45]. However, the anti-Xa assay also has some disadvantages. First, it is more costly than the aPTT test. Moreover, samples must be rapidly processed within 1 hour to prevent heparin neutralization from platelet factor 4 (PF4). In addition, since anti-thrombin plays a key role in the anti-Xa assay, patients with severe anti-thrombin deficiency display lower test values that do not reflect their actual heparin levels.
Although the anti-Xa assay is the more accurate method developed to date for measuring plasma heparin levels, this assay is not commonly used in clinical practice. Instead, the aPTT test has been used to monitor heparin infusion rates. A pioneering study conducted in 1972 with 234 patients established the current standard of using an aPTT test and ranged 1.5 to 2.5 times greater than the upper limit of the institutional reference range [6]. The aPTT test is a global test of the intrinsic coagulation pathway. Nevertheless, its results do not directly reflect the antithrombotic effects of UFH, and this test therefore has several disadvantages in the context of heparin monitoring. In contrast to the international normalized ratio (INR) of prothrombin time (PT), the aPTT test has not been standardized for heparin therapy. Many variables such as acute phase reactants, coagulation factor levels, lupus anticoagulants, and liver function affect the aPTT value. Moreover, pre-analytic stage errors that occur commonly in clinical practice, such as incorrect sample storage and short-drawn tubes, affect aPTT results [45]. Additionally, reagents and instruments have their own sensitivities. Therefore, global standards for the interpretation of aPTT results in the context of heparin therapy have not been developed, and each laboratory must set up its own heparin therapeutic range (HTR).
Here, we established the aPTT HTR corresponding to therapeutic anti-Xa levels for continuous intravenous UFH, and used appropriate monitoring to determine whether an adequate dose of UFH was applied.
Go to : 
According to the recommendations of the College of American Pathologists (CAP), we prepared ex vivo plasma samples from patients receiving intravenous (i.v.) UFH therapy [7]. Eighty-three patients were enrolled from June to September 2015 at Gyeongsang National University Hospital, Korea. Patients were diagnosed with pulmonary thromboembolism, deep vein thrombosis, arteriosclerosis obliterans, cerebral infarction, and/or ST elevation myocardial infarction. Prior to the start of heparin therapy, platelet count, aPTT, and PT values were evaluated. After the initiation of intravenous heparin administration, the anticoagulation status was monitored every 6 hours. All laboratory protocols were in accordance with CAP requirements for determining the aPTT heparin therapeutic range. Fresh blood specimens in sodium citrate bottles were obtained within 30 minutes after collection. The anti-Xa assay and the aPTT test were performed simultaneously using a STA-R Evolution (DIAGNOSTICA STAGO S.A.S., Asnières-sur-Seine, France) instrument with liquid anti-Xa and PTT A-5 (DIAGNOSTICA STAGO S.A.S.) reagents. Results were plotted on a scatterplot (X-axis, anti-Xa; Y-axis, aPTT) and linear regression analysis was performed. The best-fit line and its correlation coefficient (R2 value) were calculated using Excel 2010 (Microsoft Corporation, Redmond, WA, USA). The therapeutic aPTT range was determined by identifying the aPTT values corresponding to anti-Xa levels of 0.3 IU/mL and 0.7 IU/mL.
Go to : 
A total of 433 specimens were obtained from 83 patients. In accordance with Clinical and Laboratory Standards Institute (CLSI) guidelines, we excluded the following patients: 1) patients with incomplete baseline lab tests; 2) patients whose aPTT results were over 180 seconds, since the maximum aPTT test value in our laboratory was 180 sec; and 3) patients whose baseline PT INR level was above 1.3 [8]. All specimens with an anti-Xa level of 0 IU/mL (13.2%, 57/433) were excluded from the analysis. Only one or two samples were taken from each patient, since multiple samples from the same patient may introduce bias into the results owing to individual differences in heparin responses. After the application of the exclusion criteria, 134 samples from 71 patients were retained.
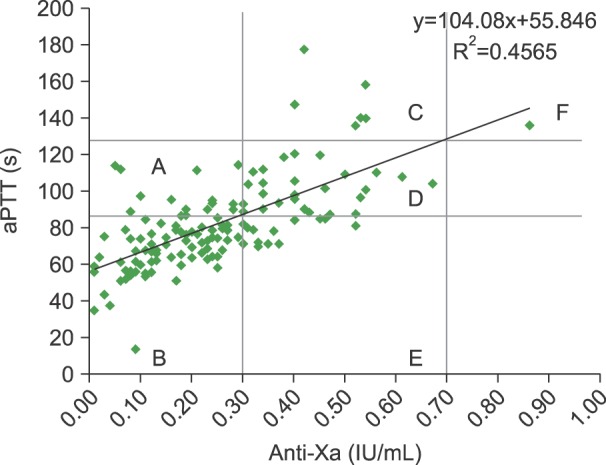
We generated a scatterplot of the aPTT and anti-Xa values (Fig. 1). The aPTT range corresponding to anti-Xa values of 0.3 IU/mL to 0.7 IU/mL, as calculated from the linear regression equation, was 87.1 sec to 128.7 sec. The R2 value of the correlation was 0.4565.
The anti-Xa levels in 65.7% (88/134) of the specimens were <0.3 IU/mL, whereas those in 33.6% (45/134) of the specimens were within the therapeutic range (0.3–0.7 IU/mL), and 0.7% (1/134) of the specimens showed levels> 0.7 IU/mL (Table 1). Similar results were obtained when HTR values projected from anti-Xa values were used. Regions B, D, and F were classified as concordant groups and regions A, C, and E were classified as discordant groups. The outcomes of the concordant and discordant groups were not significantly different. Since patients were only monitored by the aPTT test, we assumed that the discordant groups tended to receive inappropriate UFH doses and thus should have had poorer outcomes than the concordant groups, based on the assumption that the anti-Xa assay was more accurate for monitoring UFH. Analysis of 21-day mortality rates revealed that one patient with two consecutive discordant results (region E) and another with two consecutive concordant results (region B) did not survive. The patient with two consecutive discordant results was an 87-year-old man with underlying lung cancer. He had deep vein thrombosis at the left calf veins and pulmonary thromboembolism. After heparin therapy, his dyspnea improved. He was discharged after being prescribed rivaroxaban. Sixteen days later, a massive pulmonary thromboembolism occurred again and he died. The patient with two consecutive concordant results was a 79-year-old woman. A sternum fracture and pulmonary thromboembolism were noticed following a traffic accident. She received heparin therapy, but not all of her aPTT level measurements met therapeutic levels. The patients developed sudden cardiac arrest despite the attempts to resuscitate her. However, the patient number was too small to adequately compare outcomes between the two groups. No other patients experienced evident recurring thrombosis or bleeding.
Go to : 
The aPTT reference range was 29.1 sec to 43.5 sec in our laboratory. Clinicians typically employ a range that is 1.5 to 2.5 times more than the upper limit of the institutional aPTT reference range to monitor UFH therapy; in our laboratory, this range was 65.2 sec to 108.7 sec. This range is about 20 s shorter than the HTR we calculated (87.1 sec to 128.7 sec). This discrepancy may lead to infusion of subtherapeutic dosages of UFH.
Although the anti-Xa and aPTT results exhibited only a moderate correlation (R2=0.4565), it was significant enough to provide confident guidance for UFH therapy. As shown in Table 1, the fractions of subjects in each range as classified by the anti-Xa assay versus the aPTT test were comparable.
The relationship between aPTT and anti-Xa values is presented in Fig. 1. The data points in region D were within the therapeutic ranges of both the aPTT test and the anti-Xa assay. In region C, patients received an adequate amount of heparin, but the aPTT result could have caused confusion and falsely indicated the presence of an overdose. In this situation, the clinician would reduce the infusion rate. The opposite scenario holds true for region E.
The CLSI recommends an anti-Xa therapeutic reference range of 0.3 to 0.7 IU/mL, although this range is somewhat controversial [9]. Studies evaluating inter-laboratory agreement in the context of heparin monitoring have failed to show that aPTT results correlate with anti-Xa results between different hospital laboratories. Moreover, greater variation was generally observed in the anti-Xa assay compared with the aPTT test [1011]. Variations in anti-Xa measurements are observed when sample processing is delayed, as PF4 neutralizes heparin [12]. Pre-analytical errors, such as hemolysis and increase of bilirubin, also lead to the underestimation of anti-Xa levels [13]. Other coagulation factors such as antithrombin, factor II, and factor VIII may contribute to these discrepancies between the aPTT and the anti-Xa results [14]. We did not consider these factors in the present study. As a result, further studies are required to conclusively determine the optimal monitoring range for UFH therapy.
In conclusion, using a conventional aPTT target range that is 1.5 to 2.5 times greater than the upper limit of the institutional reference range may lead to inappropriate UFH dosing. Therefore, the aPTT HTR test should be carefully validated to prevent inadequate UFH dosing, and especially underdosing. A transition from the aPTT test to the anti-Xa assay is required to avoid the laborious validation of the aPTT HTR test, even though the anti-Xa assay is expensive.
Go to : 
Notes
Authors' Disclosures of Potential Conflicts of Interest: No potential conflicts of interest relevant to this article were reported.
Go to : 
References
1. Guervil DJ, Rosenberg AF, Winterstein AG, Harris NS, Johns TE, Zumberg MS. Activated partial thromboplastin time versus antifactor Xa heparin assay in monitoring unfractionated heparin by continuous intravenous infusion. Ann Pharmacother. 2011; 45:861–868. PMID: 21712506.

2. Baglin T, Barrowcliffe TW, Cohen A, Greaves M. British Committee for Standards in Haematology. Guidelines on the use and monitoring of heparin. Br J Haematol. 2006; 133:19–34. PMID: 16512825.

3. Warkentin TE, Crowther MA. Reversing anticoagulants both old and new. Can J Anaesth. 2002; 49:S11–S25. PMID: 12557411.
4. McGlasson DL, Kaczor DA, Krasuski RA, Campbell CL, Kostur MR, Adinaro JT. Effects of pre-analytical variables on the anti-activated factor X chromogenic assay when monitoring unfractionated heparin and low molecular weight heparin anticoagulation. Blood Coagul Fibrinolysis. 2005; 16:173–176. PMID: 15795534.

5. Vandiver JW, Vondracek TG. Antifactor Xa levels versus activated partial thromboplastin time for monitoring unfractionated heparin. Pharmacotherapy. 2012; 32:546–558. PMID: 22531940.

6. Basu D, Gallus A, Hirsh J, Cade J. A prospective study of the value of monitoring heparin treatment with the activated partial thromboplastin time. N Engl J Med. 1972; 287:324–327. PMID: 5041701.

7. College of American Pathologist. College of American Pathologist hematology and coagulation laboratory accreditation checklist. Northfield, IL: College of American Pathologists;2007. February 3, 2016. at http://www.cap.org/web/home/lab/accreditation/accreditation-checklists.
8. The Clinical and Laboratory Standards Institute (CLSI). One-stage prothrombin time (PT) test and activated partial thromboplastin time (APTT) test; Approved Guideline-Second edition. Wayne, PA: The Clinical and Laboratory Standards Institute;2008.
9. Garcia DA, Baglin TP, Weitz JI, Samama MM. American College of Chest Physicians. Parenteral anticoagulants: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012; 141(2 Suppl):e24S–e43S. PMID: 22315264.
10. Cuker A, Ptashkin B, Konkle BA, et al. Interlaboratory agreement in the monitoring of unfractionated heparin using the anti-factor Xa-correlated activated partial thromboplastin time. J Thromb Haemost. 2009; 7:80–86. PMID: 19017257.
11. Cuker A, Raby A, Moffat KA, Flynn G, Crowther MA. Interlaboratory variation in heparin monitoring: Lessons from the Quality Management Program of Ontario coagulation surveys. Thromb Haemost. 2010; 104:837–844. PMID: 20664895.

12. Lehman CM, Frank EL. Laboratory monitoring of heparin therapy: partial thromboplastin time or anti-Xa assay? Lab Med. 2009; 40:47–51.

13. Kostousov V, Nguyen K, Hundalani SG, Teruya J. The influence of free hemoglobin and bilirubin on heparin monitoring by activated partial thromboplastin time and anti-Xa assay. Arch Pathol Lab Med. 2014; 138:1503–1506. PMID: 25357112.

14. Takemoto CM, Streiff MB, Shermock KM, et al. Activated partial thromboplastin time and anti-xa measurements in heparin monitoring: biochemical basis for discordance. Am J Clin Pathol. 2013; 139:450–456. PMID: 23525615.
Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download