Abstract
Recent advancement in the radiotherapy technology has allowed conformal delivery of high doses of ionizing radiation precisely to the tumors while sparing large volume of the normal tissues, which have led to better clinical responses. Despite this technological advancement many advanced tumors often recur and they do so within the previously irradiated regions. How could tumors recur after receiving such high ablative doses of radiation? In this review, we outlined how radiation can elicit anti-tumor responses by introducing some of the cytokines that can be induced by ionizing radiation. We then discuss how tumor hypoxia, a major limiting factor responsible for failure of radiotherapy, may also negatively impact the anti-tumor responses. In addition, we highlight how there may be other populations of immune cells including regulatory T cells (Tregs), myeloid-derived suppressor cells (MDSCs), and tumor-associated macrophages (TAMs) that can be recruited to tumors interfering with the anti-tumor immunity. Finally, the impact of irradiation on tumor hypoxia and the immune responses according to different radiotherapy regimen is also delineated. It is indeed an exciting time to see that radiotherapy is being combined with immunotherapy in the clinic and we hope that this review can add an excitement to the field.
Since its discovery in the late 19th century by Röntgen, ionizing radiation has been utilized as one of the three (surgery, chemotherapy, and radiotherapy) most important treatment modalities for many types of cancers [1]. Ionizing radiation kills cells by inducing DNA damage, particularly DNA double-strand breaks, resulting from ionizations in or very close to the DNA [2]. Radiotherapy delivers radiation dose at a typical daily dose of around 2 Gy per fraction, 5 times a week, and up to three weeks, in a regimen called 'fractionated irradiation' [3]. Why use fractionated radiotherapy? This is because the dose that can be delivered to patients is largely limited by their normal tissue toxicity. With the lack of dose delivery technology capable of limiting normal tissue exposure, normal tissue volumes in the conventional radiotherapy have been typically much larger than the tumor volume itself [3]. Hence, the only option for delivering high tumor dose was to use fractionated irradiation regimen.
Latest technological advancement in radiotherapy including stereotactic radiosurgery (SRS) for the brain and stereotactic ablative radiotherapy (SABR) for the extracranial tissues can now deliver individual ablative high doses of radiation (15–24 Gy) to the tumor volume with a very steep dose gradient using highly conformal techniques [1]. Major advantage this technology has brought in the field of radiation oncology is the superior clinical response while significantly lowering normal tissue toxicity, due to the precise targeting ability sparing large volumes of the normal tissues [1]. However, tumors such as glioblastoma multiforme and lung cancers invariably recur and they often do so within the previously irradiated field [456].
How could tumors recur after such ablative doses of radiotherapy? Cancer cells, of course, may bear mutations in many genes, some of which are involved in intrinsic radiation sensitivity, for example, epidermal growth factor receptor (EGFR) and DNA-dependent protine kinase catalytic subunit (DNA-PKcs) [789]. Some cancers may also harbor mutations in apoptotic genes including Tumor protein 53 (TP53) or BCL2 associated X protein (BAX) which can certainly affect the tumor response to irradiation [71011]. Recently, we and others have reported that there are circulating cells, especially those bone marrow-derived cells such as myeloid cells, which can modulate the tumor response to radiotherapy [12]. In this review, we will discuss the mechanisms by which ionizing radiation induce immune responses. More specifically, anti-tumor immune responses, which should be able to bring superior clinical responses, will be summarized. Then, it will be dicussed how tumor microenvironmental factors interferes this anti-tumor immune responses.
Ionizing radiation kills cancer cells by various mechanisms of cell death, including apoptosis, necrosis, mitotic catastrophe, and immunogenic cell death [131415161718]. It has been demonstrated that both single high dose irradiation and fractionated low dose irradiation to tumors lead to the induction of damage-associated molecular pattern (DAMP) molecules [19], including high mobility group protein box 1 (HMGB1), adenosine triphosphate (ATP), heat-shock proteins (HSPs), uric acid, and interleukin-1α (IL-1α) [20] (Fig. 1). In theory, dendritic cells (DCs) should be able to take up these antigens for priming naïve T cells [21]. Furthermore, some irradiated tumors such as breast cancers have shown to release increased levels of granulocyte-macrophage colony-stimulating factor (GM-CSF) [22], the cytokine that induces DC differentiation from monocytes and hematopoietic progenitor cells (HPCs) [23]. In fact, some studies have shown that radiation facilitates DC maturation and migration [24] and an increase in tumor-reactive T cells [25] at ablative irradiation doses of 15–20 Gy. These results do suggest that ionizing radiation should bring a potent anti-tumor response. But in reality how could these anti-tumor immunity fail to trigger a potent anti-tumor response in some patients?
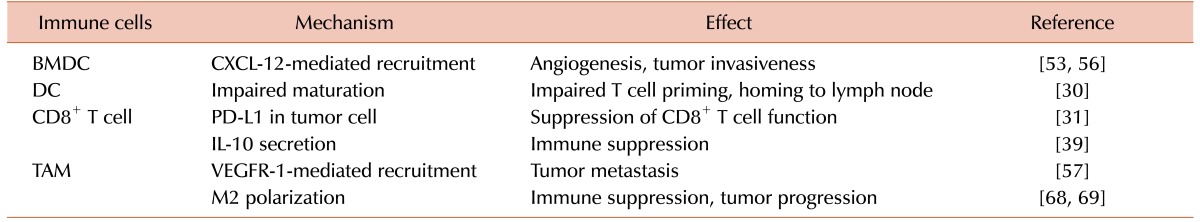
Generation of tumor-specific CD8+ T cells generally requires maturation of DCs capable of antigen uptake and presentation [132627]. This indicates that DC maturation may be severely impaired in cancer patients. It has been extensively demonstrated that tumor hypoxia is a common feature existing in many, if not all, of human and murine solid tumors [28]. Thomlinson and Gray first proposed some 50 years ago a stream of necrotic cancer cells away from the functional blood vessels in histology sections [2]. Later, Brown proposed that fluctuation in the tumor blood flow may cause temporary conditions of low oxygen tensions, leading to acute hypoxic conditions [29]. Tumor hypoxia is well known to be a major hurdle for most anti-cancer drugs because of several reasons: hypoxic cancer cells are far away from blood vessels lowering the anti-cancer drug concentrations to be delivered to those hypoxic tumor cells; hypoxic cells proliferate much slower than well-oxygenated cells escaping the cytotoxic action of many conventional anti-cancer drugs that target rapidly proliferating cells; and hypoxia acts as a selective pressure for more mutations, for example selecting cells that have lost p53-mediated apoptosis [2]. In the perspective of the effect of tumor hypoxia on the anti-tumor immunity, hypoxia is known to inhibit expression of many differentiation and maturation markers including CD1α, CD40, CD80, CD83, CD86, and major histocompatibility complex (MHC) class II molecules in response to lipopolysaccharide (LPS), the stimulatory capacity for T cell function, and DC homing to draining lymph node [30] (Table 1). Tumor hypoxia can further complicate the immune response by modulating expression of various molecules in cancer cells that are necessary for developing proper anti-tumor immunity. For example, tumor hypoxia has been reported to increase programmed death-ligand 1 (PD-L1) expression via activating hypoxia-inducible factor-1 (HIF-1) transcription factor in clear cell renal cell carcinoma [31]. PD-1 is an immune checkpoint receptor on T cell whose ligands, PD-L1 and PD-L2, are commonly being expressed in cancer cells and antigen presenting cells such as dendritic cells and macrophages [323334]. Engage of PD ligands to PD-1 leads to down-regulation of immune responses by blocking ZAP-70 phosphorylation and association with CD3-ζ [3536]. This signaling results in reducing PKC-θ activation, which activates NF-κB transcription factor leading to production of pro-inflammatory cytokines such as IL-2 [37]. Hence the use of PD-1 antibodies such as nivolumab, pembrolizumab, and pidilizumab, or PD-L1 antibodies including PD-L1BMS-936559, MPDL3280A, and MEDI-4736 are receiving much attention with a hope of much improved anti-tumor responses [38]. Hypoxia has also shown to decrease proliferation of CD8+ tumor-infiltrating lymphocytes (TILs) and induce IL-10 immunosuppressive cytokine production [39].
Abscopal effect, originally proposed by R.H. Mole in 1953, describing the effect of radiation to the distant tumor site after local irradiation within the same organism [40], is probably the ultimate proof that irradiation can trigger potent anti-tumor response systemically. It has been proposed that this is mediated by radiation-induced anti-tumor T cells [41]. A number of preclinical studies have demonstrated that addition of other strategies including Flt3 ligand [42], macrophage inflammatory protein-1α (MIP-1α) [43], or vaccine against tumor-associated antigen such as carcinoembryonic antigen (CEA) [44] can lead to tumor-specific abscopal effects by irradiation in mice. However, the fact that abscopal effect has been reported to be very rare both preclinically and clinically [20] and that the exact radiotherapy regimen responsible for such effect are not yet known suggest that much further work is needed to establish radiation-induced anti-tumor immunity.
It has been extensively reported that irradiated cancer cells or tumors including stroma produce cytokine(s) and chemokine(s) such as tumor necrosis factor-α (TNF-α), IL-1α, IL-1β, IL-6, GM-CSF, and transforming growth factor-β(TGF-β) [204546]. Released chemokines including C-C motif chemokine ligand 2 (CCL-2) and C-X-C motif chemokine ligand 12 (CXCL-12) can then act potently to recruit TILs into irradiated tumors [47]. There are many mechanisms by which ionizing radiation promotes the release of chemokines from tumors. For example, irradiation can result in upregulation of HIF either by killing aerobic cells resulting in an increase in tumor hypoxia [4849] or by reactive oxygen species (ROS) [50]-mediated inhibition of proline hydroxylase [51], the enzyme responsible for degrading HIF-α subunits [52]. The HIF transcription factor is known to be able to induce numerous cytokines, chemokines, and growth factors including CXCL-12, CCL-2 and vascular endothelial growth factor (VEGF) [535455] (Fig. 1), all of which can potently recruit TILs. The TILs express cognate receptors for many of these chemokines, for example C-X-C motif chemokine receptor-4 (CXCR-4) for CXCL-12; and C-C motif chemokine receptor-7 (CCR7) for CCL-19 or CCL-21; vascular endothelial growth factor receptor-1 (VEGFR-1) for VEGF and these interactions may further amplify the immune response. Although T-lymphocytes are also known to express CXCR-4, which in theory should be able to be recruited to CXCL-12-expressing tumors and potentiate 'anti-tumor immunity', a number of studies have shown that it is mostly myeloid cells including monocytes and macrophages that largely express CXCR-4 thereby being attracted to CXCL-12-expressing cancer cells [5657] (Fig. 1). Recruited monocytes can then reconstruct the irradiated and thereby being damaged tumor vasculature by expressing matrix metalloproteinase-9 (MMP-9) [58], S100 calciumbinding protein A8 (S100A8) chemoattractant proteins [12], or by releasing VEGF by themselves [59]. Other mechanisms by which irradiation can promote cytokine/chemokine secretion also include activation of NF-κB pathways, which results in production of various pro-inflammatory cytokines/chemokines including TNF-α, IL-1, and CXCL-12, which could then recruit TILs and induce pro-inflammatory microenvironment [60].
Recruited TILs can then further release, even higher concentrations of many of the cytokines listed above or other cytokines, such as IL-1β, IL-6, IL-10, TNF-α, and TGF-β [20]. It has been reported that CD4+ T cells are a major source for TGF-β production [61626364] and that TGF-β regulates activation of CD8+ T cells and natural killer T (NKT) cells, maintenance of peripheral Foxp3-expressing regulatory T cells (Tregs), and survival of CD4+ T cells [65]. Although TGF-β may elicit and potentiate anti-tumor CD8+ T cells, Tregs may counteract such anti-tumor activity by exerting immune suppression in co-operation with myeloid-derived suppressor cells (MDSCs) and tumor-associated macrophages (TAMs).
MDSCs and TAMs may interfere with CD8+ T cell functions in various ways. For examples, it has been shown that they express high levels of arginase-1 (Arg-1) thereby lowering arginine pool for T cell activation and responses [66]; they can also sequester cysteine thereby limiting the availability of cysteine, an amino acid essential for T cell proliferation [50]; they may destroy T cell receptors (TCRs) by producing various ROS [67].
Tumor hypoxia may play an additional role in potentiating these pro-tumor immune responses. Indeed, it has been reported that TAMs are more likely to be polarized towards M2-like pro-tumor phenotype by tumor hypoxia via activation of HIF transcription factor [6869] and that HIF can modify TAM functions such that it increases activities of Arg-1 and NADPH oxidase, which can then further compromise CD8+ cytotoxic functions towards cancer cells [50]. Both tumor hypoxia and radiation has been shown to induce epithelial mesenchymal transition (EMT) of certain cancers [707172]. EMT is a key developmental program often activated during cancer invasion and metastasis. It is currently highly controversial whether EMT triggers anti-tumor response or provokes immune evasion. Although it is possible that anti-tumor response may develop against EMT, it has been reported that SNAIL and ZEB families, transcription factors key to EMT process, are associated with increased CD4+/Foxp3+ Tregs and impaired dendritic cell functions in melanomas [73] and modulate PD-L1 in lung cancer cells [74]. Furthermore, TAMs have been reported to facilitate EMT in pancreatic cancer cells by IL-10 signaling pathway and other molecular mechanisms including increased matrix metalloproteases activities [75].
We outlined above how hypoxia may negatively impact the effect of anti-tumor immunity towards cancers. Therefore, it would be essential to understand how tumor hypoxia changes along the course of radiotherapy. It is generally believed that ionizing radiation of solid tumors would initially result in an increase in hypoxic fractions. This is because DNA radicals produced by ionizing radiation can only be permanently fixed to give rise the DNA damage that can lead to cell death only in the presence of the molecular oxygen, O2 [2]. Thus, radiation-induced cell kill would be initially confined to those of well-oxygenated cancer cells, leaving hypoxic tumor cells viable. Despite such obvious expectation, a number of recent studies indicate that this is not the case. It has been shown by using 18F-misonidazole (F-MISO) radioactive tracer and positron emission tomography (PET) to monitor the dynamic changes in intratumoral hypoxia that 10 and 20 Gy ablative dose irradiated human head and neck squamous cell carcinoma xenografts had minimal changes in the intratumoral hypoxia [76]. Our recent work with F-MISO has also demonstrated that there is no immediate increase in tumor hypoxia following 15 Gy ablative radiation [77]. Although the degree of tumor hypoxia may not change dramatically after irradiation, the extent to which each individual may have in their tumors could be quite different. More importantly, tumor hypoxia is a dynamic process in which it constantly changes spatiotemporally [78], which would affect the local immune cell functions thereby the immune responses.
Radiotherapy regimen would also influence the immune responses. Although ionizing radiation can increase MHC class I expression in a dose-dependent manner from 1 Gy up to 25 Gy and that the response could be maintained for up to 3 days [79], the anti-tumor immunity established by tumor-reactive T cells has been reported to be offset at the highest dose by an increase in Tregs [25]. Although preclinical study has reported that fractionated regimen of 7.5 Gy/fraction as optimal for induction of anti-tumor immunity [25], we have recently demonstrated that fractionated irradiation can actually deplete tumor-infiltrating T cells leading to the tumor and metastasis recurrences [80]. In a preclinical study by Lugade and colleagues [81] it was shown that a single high dose irradiation of 15 Gy is superior to fractionated (5×3 Gy) irradiation in inducing anti-tumor activities such as increased levels of antigen presenting cells and interferon-γ(IFN-γ) production in the lymph nodes. However, much further work needs to be done to investigate the detailed molecular mechanisms and immune responses associated with radiotherapy.
It is an exciting time to have a superb technical advancement in radiotherapy delivering ablative radiation doses precisely to the tumor bearing volume. Furthermore, promising clinical results of checkpoint blockades/immunotherapy further boost the initiative of combining radiotherapy with immunotherapy. In theory, this advanced technique of radiotherapy can significantly boost anti-tumor immune responses. In this review, we have outlined how tumor hypoxia, a major limiting factor contributing failures of chemotherapy and radiotherapy, can further complicate the anti-tumor immune responses. Because tumor hypoxia is a dynamic pathophysiological feature in many solid tumors, it will be essential to investigate the real-time changes in tumor hypoxia with radiotherapy and how immune cells respond towards this system. We believe this topic is of interest to not only cancer biologists, oncologists, and radiation oncologists but also numerous immunologists, which will soon bring us exciting imaging tools for monitoring dynamics of tumor hypoxia and real-time imaging of various populations of immune cells simultaneously.
References
1. Thariat J, Hannoun-Levi JM, Sun Myint A, Vuong T, Gerard JP. Past, present, and future of radiotherapy for the benefit of patients. Nat Rev Clin Oncol. 2013; 10:52–60. PMID: 23183635.

2. Brown JM, Wilson WR. Exploiting tumour hypoxia in cancer treatment. Nat Rev Cancer. 2004; 4:437–447. PMID: 15170446.

3. Timmerman R, Bastasch M, Saha D, Abdulrahman R, Hittson W, Story M. Optimizing dose and fractionation for stereotactic body radiation therapy. Normal tissue and tumor control effects with large dose per fraction. Front Radiat Ther Oncol. 2007; 40:352–365. PMID: 17641519.
4. Nakajima N, Sugawara Y, Kataoka M, et al. Differentiation of tumor recurrence from radiation-induced pulmonary fibrosis after stereotactic ablative radiotherapy for lung cancer: characterization of 18F-FDG PET/CT findings. Ann Nucl Med. 2013; 27:261–270. PMID: 23299492.

5. Minniti G, Amelio D, Amichetti M, et al. Patterns of failure and comparison of different target volume delineations in patients with glioblastoma treated with conformal radiotherapy plus concomitant and adjuvant temozolomide. Radiother Oncol. 2010; 97:377–381. PMID: 20855119.

6. Senthi S, Lagerwaard FJ, Haasbeek CJ, Slotman BJ, Senan S. Patterns of disease recurrence after stereotactic ablative radiotherapy for early stage non-small-cell lung cancer: a retrospective analysis. Lancet Oncol. 2012; 13:802–809. PMID: 22727222.

7. Ahn GO, Brown JM. Influence of bone marrow-derived hematopoietic cells on the tumor response to radiotherapy: experimental models and clinical perspectives. Cell Cycle. 2009; 8:970–976. PMID: 19270527.

8. Gerweck LE, Vijayappa S, Kurimasa A, Ogawa K, Chen DJ. Tumor cell radiosensitivity is a major determinant of tumor response to radiation. Cancer Res. 2006; 66:8352–8355. PMID: 16951142.

9. Liang K, Ang KK, Milas L, Hunter N, Fan Z. The epidermal growth factor receptor mediates radioresistance. Int J Radiat Oncol Biol Phys. 2003; 57:246–254. PMID: 12909240.

10. Burdelya LG, Komarova EA, Hill JE, et al. Inhibition of p53 response in tumor stroma improves efficacy of anticancer treatment by increasing antiangiogenic effects of chemotherapy and radiotherapy in mice. Cancer Res. 2006; 66:9356–9361. PMID: 17018587.

11. Garcia-Barros M, Paris F, Cordon-Cardo C, et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science. 2003; 300:1155–1159. PMID: 12750523.

12. Ahn GO, Tseng D, Liao CH, Dorie MJ, Czechowicz A, Brown JM. Inhibition of Mac-1 (CD11b/CD18) enhances tumor response to radiation by reducing myeloid cell recruitment. Proc Natl Acad Sci U S A. 2010; 107:8363–8368. PMID: 20404138.

13. Wattenberg MM, Fahim A, Ahmed MM, Hodge JW. Unlocking the combination: potentiation of radiation-induced antitumor responses with immunotherapy. Radiat Res. 2014; 182:126–138. PMID: 24960415.

14. Dewey WC, Ling CC, Meyn RE. Radiation-induced apoptosis: relevance to radiotherapy. Int J Radiat Oncol Biol Phys. 1995; 33:781–796. PMID: 7591884.

15. Verheij M, Bartelink H. Radiation-induced apoptosis. Cell Tissue Res. 2000; 301:133–142. PMID: 10928286.

16. Bhattathiri NV, Bindu L, Remani P, Chandralekha B, Nair KM. Radiation-induced acute immediate nuclear abnormalities in oral cancer cells: serial cytologic evaluation. Acta Cytol. 1998; 42:1084–1090. PMID: 9755662.
17. Eriksson D, Lofroth PO, Johansson L, Riklund KA, Stigbrand T. Cell cycle disturbances and mitotic catastrophes in HeLa Hep2 cells following 2.5 to 10 Gy of ionizing radiation. Clin Cancer Res. 2007; 13:5501s–5508s. PMID: 17875782.

18. Golden EB, Pellicciotta I, Demaria S, Barcellos-Hoff MH, Formenti SC. The convergence of radiation and immunogenic cell death signaling pathways. Front Oncol. 2012; 2:88. PMID: 22891162.

19. Ahmed MM, Guha C, Hodge JW, Jaffee E. Immunobiology of radiotherapy: new paradigms. Radiat Res. 2014; 182:123–125. PMID: 25036983.

20. Lumniczky K, Safrany G. The impact of radiation therapy on the antitumor immunity: local effects and systemic consequences. Cancer Lett. 2015; 356:114–125. PMID: 23994343.

21. Gallo PM, Gallucci S. The dendritic cell response to classic, emerging, and homeostatic danger signals. Implications for autoimmunity. Front Immunol. 2013; 4:138. PMID: 23772226.

22. Vilalta M, Rafat M, Giaccia AJ, Graves EE. Recruitment of circulating breast cancer cells is stimulated by radiotherapy. Cell Rep. 2014; 8:402–409. PMID: 25017065.

23. van de Laar L, Coffer PJ, Woltman AM. Regulation of dendritic cell development by GM-CSF: molecular control and implications for immune homeostasis and therapy. Blood. 2012; 119:3383–3393. PMID: 22323450.

24. Lee Y, Auh SL, Wang Y, et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood. 2009; 114:589–595. PMID: 19349616.

25. Schaue D, Ratikan JA, Iwamoto KS, McBride WH. Maximizing tumor immunity with fractionated radiation. Int J Radiat Oncol Biol Phys. 2012; 83:1306–1310. PMID: 22208977.

26. Rock KL. A new foreign policy: MHC class I molecules monitor the outside world. Immunol Today. 1996; 17:131–137. PMID: 8820271.

27. Melief CJ. Mini-review: Regulation of cytotoxic T lymphocyte responses by dendritic cells: peaceful coexistence of cross-priming and direct priming? Eur J Immunol. 2003; 33:2645–2654. PMID: 14515248.

28. Brown JM, Giaccia AJ. The unique physiology of solid tumors: opportunities (and problems) for cancer therapy. Cancer Res. 1998; 58:1408–1416. PMID: 9537241.
29. Brown JM. Evidence for acutely hypoxic cells in mouse tumours, and a possible mechanism of reoxygenation. Br J Radiol. 1979; 52:650–656. PMID: 486895.

30. Mancino A, Schioppa T, Larghi P, et al. Divergent effects of hypoxia on dendritic cell functions. Blood. 2008; 112:3723–3734. PMID: 18694997.

31. Ruf M, Moch H, Schraml P. PD-L1 expression is regulated by hypoxia inducible factor in clear cell renal cell carcinoma. Int J Cancer. 2016; 139:396–403. PMID: 26945902.

32. Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002; 8:793–800. PMID: 12091876.

33. Ishida M, Iwai Y, Tanaka Y, et al. Differential expression of PD-L1 and PD-L2, ligands for an inhibitory receptor PD-1, in the cells of lymphohematopoietic tissues. Immunol Lett. 2002; 84:57–62. PMID: 12161284.

34. Flies DB, Chen L. The new B7s: playing a pivotal role in tumor immunity. J Immunother. 2007; 30:251–260. PMID: 17414316.

35. Sheppard KA, Fitz LJ, Lee JM, et al. PD-1 inhibits T-cell receptor induced phosphorylation of the ZAP70/CD3zeta signalosome and downstream signaling to PKCtheta. FEBS Lett. 2004; 574:37–41. PMID: 15358536.
36. Yokosuka T, Takamatsu M, Kobayashi-Imanishi W, Hashimoto-Tane A, Azuma M, Saito T. Programmed cell death 1 forms negative costimulatory microclusters that directly inhibit T cell receptor signaling by recruiting phosphatase SHP2. J Exp Med. 2012; 209:1201–1217. PMID: 22641383.

37. Lin X, O'Mahony A, Mu Y, Geleziunas R, Greene WC. Protein kinase C-theta participates in NF-kappaB activation induced by CD3-CD28 costimulation through selective activation of IkappaB kinase beta. Mol Cell Biol. 2000; 20:2933–2940. PMID: 10733597.
38. Philips GK, Atkins M. Therapeutic uses of anti-PD-1 and anti-PD-L1 antibodies. Int Immunol. 2015; 27:39–46. PMID: 25323844.

39. Vuillefroy de Silly R, Ducimetiere L, Yacoub Maroun C, Dietrich PY, Derouazi M, Walker PR. Phenotypic switch of CD8(+) T cells reactivated under hypoxia toward IL-10 secreting, poorly proliferative effector cells. Eur J Immunol. 2015; 45:2263–2275. PMID: 25929785.
40. Mole RH. Whole body irradiation; radiobiology or medicine? Br J Radiol. 1953; 26:234–241. PMID: 13042090.
41. Demaria S, Formenti SC. Can abscopal effects of local radiotherapy be predicted by modeling T cell trafficking? J Immunother Cancer. 2016; 4:29. PMID: 27190630.

42. Demaria S, Ng B, Devitt ML, et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys. 2004; 58:862–870. PMID: 14967443.

43. Shiraishi K, Ishiwata Y, Nakagawa K, et al. Enhancement of antitumor radiation efficacy and consistent induction of the abscopal effect in mice by ECI301, an active variant of macrophage inflammatory protein-1alpha. Clin Cancer Res. 2008; 14:1159–1166. PMID: 18281550.
44. Hodge JW, Sharp HJ, Gameiro SR. Abscopal regression of antigen disparate tumors by antigen cascade after systemic tumor vaccination in combination with local tumor radiation. Cancer Biother Radiopharm. 2012; 27:12–22. PMID: 22283603.

45. Zhang JS, Nakatsugawa S, Niwa O, Ju GZ, Liu SZ. Ionizing radiation-induced IL-1 alpha, IL-6 and GM-CSF production by human lung cancer cells. Chin Med J (Engl). 1994; 107:653–657. PMID: 7805455.
46. Yamanaka R, Tanaka R, Yoshida S. Effects of irradiation on cytokine production in glioma cell lines. Neurol Med Chir (Tokyo). 1993; 33:744–748. PMID: 7506809.

47. Dunn GP, Dunn IF, Curry WT. Focus on TILs: Prognostic significance of tumor infiltrating lymphocytes in human glioma. Cancer Immun. 2007; 7:12. PMID: 17691714.
48. Moeller BJ, Cao Y, Li CY, Dewhirst MW. Radiation activates HIF-1 to regulate vascular radiosensitivity in tumors: role of reoxygenation, free radicals, and stress granules. Cancer Cell. 2004; 5:429–441. PMID: 15144951.
49. Semenza GL. Intratumoral hypoxia, radiation resistance, and HIF-1. Cancer Cell. 2004; 5:405–406. PMID: 15144945.

50. Srivastava MK, Sinha P, Clements VK, Rodriguez P, Ostrand-Rosenberg S. Myeloid-derived suppressor cells inhibit T-cell activation by depleting cystine and cysteine. Cancer Res. 2010; 70:68–77. PMID: 20028852.

51. Pan Y, Mansfield KD, Bertozzi CC, et al. Multiple factors affecting cellular redox status and energy metabolism modulate hypoxiainducible factor prolyl hydroxylase activity in vivo and in vitro. Mol Cell Biol. 2007; 27:912–925. PMID: 17101781.

52. Schofield CJ, Ratcliffe PJ. Oxygen sensing by HIF hydroxylases. Nat Rev Mol Cell Biol. 2004; 5:343–354. PMID: 15122348.

53. Du R, Lu KV, Petritsch C, et al. HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell. 2008; 13:206–220. PMID: 18328425.
54. Ceradini DJ, Kulkarni AR, Callaghan MJ, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004; 10:858–864. PMID: 15235597.

55. Mojsilovic-Petrovic J, Callaghan D, Cui H, Dean C, Stanimirovic DB, Zhang W. Hypoxia-inducible factor-1 (HIF-1) is involved in the regulation of hypoxia-stimulated expression of monocyte chemoattractant protein-1 (MCP-1/CCL2) and MCP-5 (Ccl12) in astrocytes. J Neuroinflammation. 2007; 4:12. PMID: 17474992.

56. Kioi M, Vogel H, Schultz G, Hoffman RM, Harsh GR, Brown JM. Inhibition of vasculogenesis, but not angiogenesis, prevents the recurrence of glioblastoma after irradiation in mice. J Clin Invest. 2010; 120:694–705. PMID: 20179352.

57. Hiratsuka S, Duda DG, Huang Y, et al. C-X-C receptor type 4 promotes metastasis by activating p38 mitogen-activated protein kinase in myeloid differentiation antigen (Gr-1)-positive cells. Proc Natl Acad Sci U S A. 2011; 108:302–307. PMID: 21173223.

58. Ahn GO, Brown JM. Matrix metalloproteinase-9 is required for tumor vasculogenesis but not for angiogenesis: role of bone marrow-derived myelomonocytic cells. Cancer Cell. 2008; 13:193–205. PMID: 18328424.

59. Ahn GO, Seita J, Hong BJ, et al. Transcriptional activation of hypoxia-inducible factor-1 (HIF-1) in myeloid cells promotes angiogenesis through VEGF and S100A8. Proc Natl Acad Sci U S A. 2014; 111:2698–2703. PMID: 24497508.

60. Richmond A. Nf-kappa B, chemokine gene transcription and tumour growth. Nat Rev Immunol. 2002; 2:664–674. PMID: 12209135.
61. Haneda K, Sano K, Tamura G, et al. Transforming growth factor-beta secreted from CD4(+) T cells ameliorates antigeninduced eosinophilic inflammation. A novel high-dose tolerance in the trachea. Am J Respir Cell Mol Biol. 1999; 21:268–274. PMID: 10423411.
62. Ouyang W, Oh SA, Ma Q, Bivona MR, Zhu J, Li MO. TGF-beta cytokine signaling promotes CD8+ T cell development and low-affinity CD4+ T cell homeostasis by regulation of interleukin-7 receptor alpha expression. Immunity. 2013; 39:335–346. PMID: 23932572.
63. Doisne JM, Bartholin L, Yan KP, et al. iNKT cell development is orchestrated by different branches of TGF-beta signaling. J Exp Med. 2009; 206:1365–1378. PMID: 19451264.
64. Li MO, Flavell RA. TGF-beta: a master of all T cell trades. Cell. 2008; 134:392–404. PMID: 18692464.
65. Li MO, Sanjabi S, Flavell RA. Transforming growth factor-beta controls development, homeostasis, and tolerance of T cells by regulatory T cell-dependent and -independent mechanisms. Immunity. 2006; 25:455–471. PMID: 16973386.
66. Doedens AL, Stockmann C, Rubinstein MP, et al. Macrophage expression of hypoxia-inducible factor-1 alpha suppresses T-cell function and promotes tumor progression. Cancer Res. 2010; 70:7465–7475. PMID: 20841473.
67. Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012; 12:253–268. PMID: 22437938.

68. Laoui D, Van Overmeire E, Di Conza G, et al. Tumor hypoxia does not drive differentiation of tumor-associated macrophages but rather fine-tunes the M2-like macrophage population. Cancer Res. 2014; 74:24–30. PMID: 24220244.

69. Henze AT, Mazzone M. The impact of hypoxia on tumor-associated macrophages. J Clin Invest. 2016; [Epub ahead of print].

70. Kawamoto A, Yokoe T, Tanaka K, et al. Radiation induces epithelial-mesenchymal transition in colorectal cancer cells. Oncol Rep. 2012; 27:51–57. PMID: 21971767.

71. Yan S, Wang Y, Yang Q, et al. Low-dose radiation-induced epithelial-mesenchymal transition through NF-kappaB in cervical cancer cells. Int J Oncol. 2013; 42:1801–1806. PMID: 23483258.
72. He E, Pan F, Li G, Li J. Fractionated ionizing radiation promotes epithelial-mesenchymal transition in human esophageal cancer cells through PTEN deficiency-mediated akt activation. PLoS One. 2015; 10:e0126149. PMID: 26000878.

73. Kudo-Saito C, Shirako H, Takeuchi T, Kawakami Y. Cancer metastasis is accelerated through immunosuppression during Snail-induced EMT of cancer cells. Cancer Cell. 2009; 15:195–206. PMID: 19249678.

74. Chen L, Gibbons DL, Goswami S, et al. Metastasis is regulated via microRNA-200/ZEB1 axis control of tumour cell PD-L1 expression and intratumoral immunosuppression. Nat Commun. 2014; 5:5241. PMID: 25348003.

75. Liu CY, Xu JY, Shi XY, et al. M2-polarized tumor-associated macrophages promoted epithelial-mesenchymal transition in pancreatic cancer cells, partially through TLR4/IL-10 signaling pathway. Lab Invest. 2013; 93:844–854. PMID: 23752129.

76. Fatema CN, Zhao S, Zhao Y, et al. Dual tracer evaluation of dynamic changes in intratumoral hypoxic and proliferative states after radiotherapy of human head and neck cancer xenografts using radiolabeled FMISO and FLT. BMC Cancer. 2014; 14:692. PMID: 25245041.

77. Song C, Hong BJ, Bok S, et al. Real-time tumor oxygenation changes after single high-dose radiation therapy in orthotopic and subcutaneous lung cancer in mice: Clinical implication for stereotactic ablative radiation therapy schedule optimization. Int J Radiat Oncol Biol Phys. 2016; 95:1022–1031. PMID: 27130790.
78. Lin Z, Mechalakos J, Nehmeh S, et al. The influence of changes in tumor hypoxia on dose-painting treatment plans based on 18F-FMISO positron emission tomography. Int J Radiat Oncol Biol Phys. 2008; 70:1219–1228. PMID: 18313529.

79. Reits EA, Hodge JW, Herberts CA, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med. 2006; 203:1259–1271. PMID: 16636135.

80. Filatenkov A, Baker J, Muller AM, et al. Treatment of 4T1 metastatic breast cancer with combined hypofractionated irradiation and autologous T-cell infusion. Radiat Res. 2014; 182:163–169. PMID: 24992165.

81. Lugade AA, Moran JP, Gerber SA, Rose RC, Frelinger JG, Lord EM. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol. 2005; 174:7516–7523. PMID: 15944250.

Fig. 1
Diagram outlining how ionizing radiation (IR) of tumors leads to anti-tumor responses and how tumor hypoxia can interfere such loop. (A) Ionizing radiation can induce anti-tumor immunity via secreting various danger-associated molecular pattern (DAMP) molecules, which can stimulate dendritic cells and cytotoxic T cells. (B) However tumor hypoxia can mediate various pathways, in which can counteract the antitumor immunity.
Abbreviations: DCs, dendritic cells; HMGB1, high mobility group protein box 1; ATP, adenosine triphosphate; HSPs, heat shock proteins; HIF-1, hypoxia-inducible factor-1; PD-L1, programmed death-ligand 1; VEGF, vascular endothelial growth factor; VEGFR-1, vascular endothelial growth factor receptor-1; CXCL-12, C-X-C motif chemokine ligand 12; CXCR-4, C-X-C motif chemokine receptor-4; MDSCs, myeloid-derived suppressor cells; TAMs, tumor-associated macrophages; MMP, matrix metalloproteinase; S100A8, S100 calcium-binding protein A8; IL-1β, interleukin-1β; IL-6, interleukin-6; TNF-α, tumor necrosis factor-α.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download