TO THE EDITOR: Macrolide antibiotics (Macs) have been proposed as potential antineoplastic and immunomodulatory agents in addition to their primary role as antibacterial agents. They have anti-lymphoproliferative effects and are clinically applicable for treating lymphoid malignancies. Several previous reports have indicated that B-cell malignancies can be treated successfully with Macs [123]. With regard to T-cell lineage malignancies, we have reported previously a case of mixed phenotype acute leukemia, T/myeloid, not otherwise specified, treated successfully using clarithromycin (CAM), a Mac, in combination with prednisolone (PSL) [4]. Here, we report the first case of angioimmunoblastic T-cell lymphoma (AITL) treated successfully with CAM in combination with PSL.
A 71-year-old man with hepatic cell carcinoma was admitted with fever, a dull feeling in the throat, and bilateral cervical lymph node swellings that had grown gradually. Physical examination showed deformation of the palatopharyngeal arch and several swollen lymph nodes in the bilateral cervical region. However, no other superficial swollen lymph nodes were detected. Neither articular swellings nor skin rashes were found. Laboratory findings were as follows: white blood cell count, 3.40×103/µL; hemoglobin, 13.2 g/dL; platelet count, 91,000/µL; total protein level, 7.1 g/dL; albumin level, 4.0 g/dL; C-reactive protein level, 1.05 mg/dL; immunoglobulin (Ig)G level, 1,587 mg/dL; IgM level, 235 mg/dL; IgA level, 198 mg/dL. The antinuclear antibody titer was ×40. Hepatitis B surface antigen was negative; however, anti-hepatitis C antibody was positive. The Epstein-Barr virus antibody titer (fluorescent antibody technique), viral capsid antigen-IgG and –IgM levels, and Epstein-Barr nuclear antigen levels were ×320, less than ×10, and ×40, respectively. Serum soluble interleukin-2 receptor (sIL-2R) levels, that are closely related to disease activity, increased to 2,239 U/mL (normal, <500 U/mL).
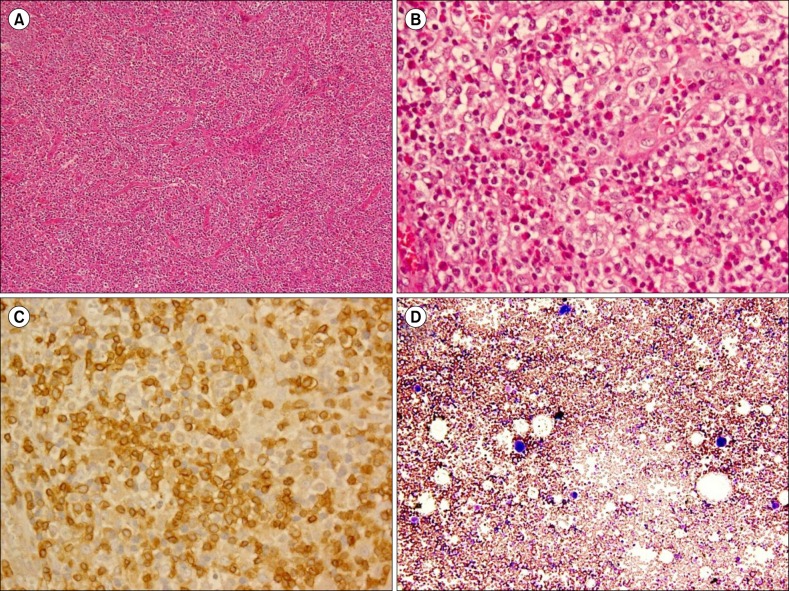
A cervical computed tomography (CT) scan revealed right cervical lymphadenopathy (Fig. 1A). Chest and abdominal CT revealed neither mediastinal nor para-aortic lymphadenopathy. An abdominal CT revealed a slightly contracted liver and splenomegaly. Right cervical lymph node biopsy specimens showed effacement of the normal architecture with abundant pleomorphic cells and marked proliferation of arborizing high endothelial venules (Fig. 2A). Pleomorphic cells were composed of lymphocytes, eosinophils, and atypical cells with intermediate-sized nuclei and clear cytoplasm (Fig. 2B). Immunohistochemically, atypical cells were CD3+ (Fig. 2C) and CD4+ (data not shown), which indicated a T-cell lineage. However, pleomorphic cells, including these atypical cells, were negative for Epstein-Barr virus using in situ hybridization.
A molecular study performed on the rearrangement of T-cell receptors revealed polyclonality. An IgH gene rearrangement study also showed polyclonality. Cytogenetic analysis from 20 mitotic cells in biopsy specimens revealed 46, XY (15/20), 45, X,-Y (1/20), and 47, XY, +5 (4/20). A bone marrow aspiration smear showed no abnormal cells. Based on these findings, a diagnosis of AITL was made. Because cervical lymph nodes had enlarged gradually before the diagnosis was made, the patient was unsuccessfully treated with PSL (50 mg/day). After the diagnosis was made, there was no time to determine the staging of lymphoma because of the gradual exacerbation of the patient's general condition. Therefore, we decided to initiate chemotherapy. After 3 cycles of chemotherapy with tetrahydropyranyl doxorubicin (50 mg on day 1), cyclophosphamide (CPM) (750 mg on day 1), vincristine (1.6 mg on day 1), and PSL (50 mg on days 1-5), right cervical lymphadenopathy improved considerably as shown by the enhanced CT findings (Fig. 1B). sIL-2R levels decreased to 1,049 U/mL, although leukopenia and thrombocytopenia appeared.
Three weeks after chemotherapy, leukopenia improved; however, platelet counts remained at approximately 30,000/µL. The patient was treated with PSL (20 mg/day followed by 10 mg/day) as an alternative to the above-mentioned chemotherapy. Five weeks after treatment, enlargement of the cervical lymph nodes was not evident by manual palpation; however, platelet counts decreased to 9,000/µL with sIL-2R levels increasing to 2,090 U/mL. The patient then received a platelet transfusion for severe thrombocytopenia. A bone marrow aspiration smear revealed normal bone marrow with a nucleated cell count of 80,000/µL and a megakaryocyte count of 48/µL without dysplasia and hemophagocytosis (Fig. 2D).
Examinations for viral infections, including parvovirus and cytomegalovirus IgM antibodies, were negative. The patient was positive for anti-Helicobacter
(H) pylori IgG antibodies. Because thrombocytopenia worsened along with the elevated sIL-2R levels, the patient was diagnosed with AITL-associated immune thrombocytopenia (ITP) rather than H. pylori-positive ITP. The patient chose not to receive the above-mentioned chemotherapy regimen and was unsuccessfully treated with CPM (50 mg/day) in combination with PSL (10 mg/day) for 1 week. After CPM was stopped, CAM (800 mg/day) was added to PSL (10 mg/day) in expectation of its immunomodulatory and antilymphoproliferative effects. Two weeks after CAM add-on therapy, platelet counts began to increase, reaching 48,000/µL at 6 weeks. Six months after CAM add-on therapy, the right cervical lymphadenopathy completely resolved based on the CT findings (Fig. 1C). The sIL-2R level decreased to 467 U/mL and the platelet count increased to 63,000/µL. Nine months after the initiation of CAM add-on therapy, the recurrence of lymphadenopathy was not recognized based on CT findings and platelet counts remained at approximately 80,000/µL.
AITL is a peripheral T-cell lymphoma clinically characterized by lymphadenopathy, hepatosplenomegaly, and skin lesions. Laboratory data often reveal various hematological, biochemical, and/or immunological abnormalities. Anemia (often hemolytic and Coombs-positive), polyclonal hypergammaglobulinemia, and hypereosinophilia are the most common alterations. Other common findings include lymphopenia, thrombocytopenia, and the presence of various autoantibodies [5]. The clinical outcome of AITL managed with conventional lymphoma protocols remains poor, with a median survival of <36 months and a 5-year survival rate of 30-35%. The majority of mortality associated with AITL is a consequence of profound immune deregulation rather than tumor bulk. Therefore, several cases of successful treatment with immunomodulatory agents, such as cyclosporine (CS) and thalidomide, have been reported [6]. Although the precise mechanism accounting for the efficacy of CS in treating AITL remains unclear, it has been speculated that CS exerts an antilymphoma effect through its direct antitumor activity by inhibiting the calcineurin-nuclear factor of the activated T-cell transcription factor pathway, and shutting down cytokine storms induced by AITL [7]. With regard to thalidomide, its effects include the downregulation of proinflammatory cytokines, enhanced CD8-positive T-cell responses, alterations in the expression of cell adhesion molecules, and reductions in prostaglandins [6].
In the present case, conventional chemotherapy was effective; however, it could not be continued because of cytopenia and the patient's preference. PSL was administered as an alternative, but it was unsuccessful. Consequently, AITL-associated ITP appeared. CPM was administered, but was also unsuccessful.
We reported previously that Macs were effective for treating primary ITP regardless of H. pylori infection. Their effectiveness was thought to be attributable, at least in part, to the immunomodulatory effects of Macs [8]. Moreover, we reported that CAM was effective for malignancies with a T-cell lineage [4]. Therefore, CAM was administered in expectation of its immunomodulatory and antilymphoproliferative effects. Consequently, not only platelet counts but also cervical lymphadenopathy improved.
Macs are considered to be potential antineoplastic and immunomodulatory agents. Macs, such as CAM, have been found recently to exhibit antiproliferative activities against B-cell lymphomas [123]. Ishimatsu et al. [1] reported two cases of pulmonary mucosa-associated lymphoid tissue lymphoma successfully treated using CAM. They speculated that CAM induced apoptosis of lymphocytes via down-regulation of the antiapoptotic protein Bcl-xL. We also reported previously a case of follicular B-cell lymphoma successfully treated using CAM [2]. Because CAM is known to downregulate bcl-2, we speculated that it induced apoptosis of lymphoma cells.
Erythromycin, a Mac, has immunomodulatory effects, presumably via an interaction with nuclear factor-κB expression, inhibiting T-cell proliferation and inducing T-cell apoptosis [9]. Recently, another Mac (azithromycin), and to a lesser extent CAM, have been reported to act as immunosuppressive agents on CD4+ T-cells by inhibiting mammalian target of rapamycin activity [10]. Based on these data, the effectiveness of CAM for AITL in the present case may have been attributable to its immunomodulatory activity, which is a type of antineoplastic effect.
Vascular endothelial growth factor (VEGF) is an inducer of angiogenesis and lymphangiogenesis through its activity as a highly specific mitogen for endothelial cells [11]. AILT shows high levels of VEGF expression on both lymphoma and endothelial cells, and is the most prominent vascular component among lymphomas. In addition, VEGF expression is related to the progression of lymphoma [12]. Several cases of successful treatment of AITL have been reported using bevacizumab, a monoclonal anti-VEGF antibody [13]. Inhibition of VEGF mRNA levels by the Macs CAM and roxithromycin has been reported [14]. The results of a recent randomized trial in non-Hodgkin lymphoma indicated that the addition of CAM to a CVP (CPM, vincristine, and PSL) regimen reduced soluble VEGF levels more than CVP alone. Therefore, the effect of CAM appears to involve the VEGF pathway [15]. Based on these data, the effectiveness of CAM treatment in the present case may have been partly associated with the inhibitory effects of CAM on VEGF production.
Because we are only reporting on a single case, more research is necessary before CAM treatment can be adopted widely.
References
1. Ishimatsu Y, Mukae H, Matsumoto K, et al. Two cases with pulmonary mucosa-associated lymphoid tissue lymphoma successfully treated with clarithromycin. Chest. 2010; 138:730–733. PMID: 20822996.

2. Ohe M, Hashino S. A case of follicular B-cell lymphoma treated using clarithromycin. Korean J Hematol. 2011; 46:203–206. PMID: 22065978.

3. Ohe M, Hashino S, Hattori A. Successful treatment of diffuse large B-cell lymphoma with clarithromycin and prednisolone. Korean J Hematol. 2012; 47:293–297. PMID: 23320009.

4. Ohe M, Hashino S. Successful treatment with clarithromycin for Mixed phenotype acute leukemia, T/myeloid, NOS. Rinsho Ketsueki. 2010; 51:297–299. PMID: 20467229.
5. de Leval L, Gisselbrecht C, Gaulard P. Advances in the understanding and management of angioimmunoblastic T-cell lymphoma. Br J Haematol. 2010; 148:673–689. PMID: 19961485.

6. Dogan A, Ngu LS, Ng SH, Cervi PL. Pathology and clinical features of angioimmunoblastic T-cell lymphoma after successful treatment with thalidomide. Leukemia. 2005; 19:873–875. PMID: 15744336.

7. Matsumura Y, Kuroda J, Shimura Y, et al. Cyclosporine A and reduced-intensity conditioning allogeneic stem cell transplantation for relapsed angioimmunoblastic T cell lymphoma with hemophagocytic syndrome. Intern Med. 2012; 51:2785–2787. PMID: 23037475.

8. Ohe M, Hashino S. Successful treatment with erythromycin for idiopathic thrombocytopenic purpura. Korean J Hematol. 2011; 46:139–142. PMID: 21747888.

9. Wu L, Zhang W, Tian L, Bao K, Li P, Lin J. Immunomodulatory effects of erythromycin and its derivatives on human T-lymphocyte in vitro. Immunopharmacol Immunotoxicol. 2007; 29:587–596. PMID: 18075867.
10. Ratzinger F, Haslacher H, Poeppl W, et al. Azithromycin suppresses CD4(+) T-cell activation by direct modulation of mTOR activity. Sci Rep. 2014; 4:7438. PMID: 25500904.

11. Hoeben A, Landuyt B, Highley MS, Wildiers H, Van Oosterom AT, De Bruijn EA. Vascular endothelial growth factor and angiogenesis. Pharmacol Rev. 2004; 56:549–580. PMID: 15602010.

12. Zhao WL, Mourah S, Mounier N, et al. Vascular endothelial growth factor-A is expressed both on lymphoma cells and endothelial cells in angioimmunoblastic T-cell lymphoma and related to lymphoma progression. Lab Invest. 2004; 84:1512–1519. PMID: 15311211.

13. Aguiar Bujanda D. Complete response of relapsed angioimmunoblastic T-cell lymphoma following therapy with bevacizumab. Ann Oncol. 2008; 19:396–397. PMID: 18245132.

14. Matsune S, Sun D, Ohori J, et al. Inhibition of vascular endothelial growth factor by macrolides in cultured fibroblasts from nasal polyps. Laryngoscope. 2005; 115:1953–1956. PMID: 16319604.

15. Saad AS, Shaheen SM, Elhamamsy MH, Badary OA. An open-label randomized controlled phase II study of clarithromycin (CL) plus CVP in patients (pts) with previously untreated stage III/IV indolent non Hodgkin lymphoma (NHL). J Clin Oncol (ASCO Annual Meeting Abstracts). 2014; 32(Suppl):abst e19510.

Fig. 1
Cervical computed tomography (CT) images. (A) Cervical CT reveals right cervical lymphadenopathy. (B) Cervical enhanced CT reveals considerable improvement in right cervical lymphadenopathy after 3 cycles of chemotherapy with tetrahydropyranyl doxorubicin (50 mg on day 1), cyclophosphamide (750 mg on day 1), vincristine (1.6 mg on day 1), and prednisolone (50 mg on days 1–5). (C) Cervical CT reveals complete resolution of the right cervical lymphadenopathy at 6 months after clarithromycin add-on therapy.
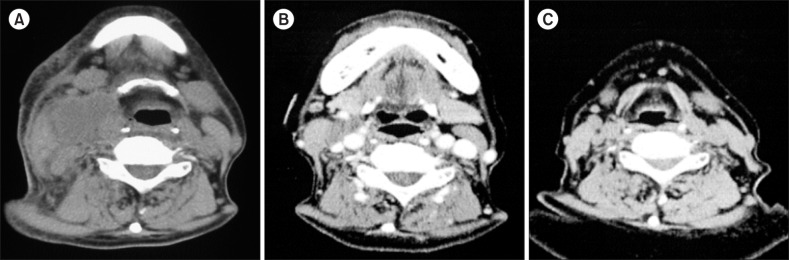
Fig. 2
Cervical histopathology and immunohistochemical images. (A) A right cervical lymph node biopsy specimen reveals effacement of the normal architecture with abundant pleomorphic cells and marked proliferation of arborizing high endothelial venules (hematoxylin and eosin stain, ×100). (B) Pleomorphic cells composed of lymphocytes, eosinophils, and atypical cells with intermediate-sized nuclei and clear cytoplasm are evident (hematoxylin and eosin stain, ×400). (C) Immunohistochemical examination shows CD3 positive staining in lymphocytes and atypical cells (×400). (D) A bone marrow aspiration smear reveals normal bone marrow (May-Giemsa stain, ×100).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download