Abstract
Background
A high Ki-67 proliferation index (PI) in neoplastic cells is associated with poor survival in mantle cell lymphoma (MCL). We aimed to determine the cut-off values for the Ki-67 PI as a prognostic factor in MCL according to bone marrow findings.
Methods
Immunohistochemical (IHC) staining for Ki-67 was performed on formalin-fixed paraffin-embedded biopsy tissues from 56 patients with MCL. Patients were grouped based on their Ki-67 PI values. Survival analyses were carried out and the cut-off value for the Ki-67 PI was determined.
Results
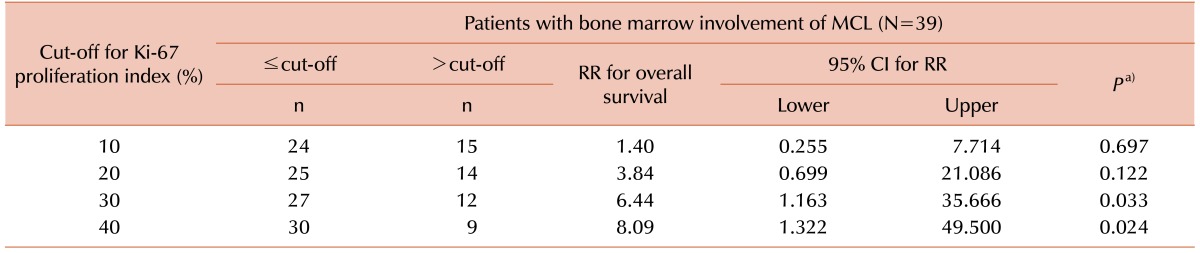
Of the 56 patients, 39 (69.6%) showed bone marrow involvement of MCL; 21 of these patients had leukemic manifestations at the time of diagnosis. The results of the Ki-67 IHC staining were as follows: ≤10% in 22 patients, 11–20% in 14 patients, 21–30% in 3 patients, 31–40% in 4 patients, 41–50% in 4 patients, and >50% in 9 patients. A cut-off value of 20% revealed significantly different survival rates with mean survival times of 69.8 months (Ki-67 PI≤20%) and 47.9 months (Ki-67 PI>20%), irrespective of bone marrow findings (P=0.034). Clinical outcomes did not differ, regardless of bone marrow findings. However, in cases with bone marrow involvement, the Ki-67 cut-off value of 30% for overall survival was required to yield statistical significance (P=0.033).
Mantle cell lymphoma (MCL) is a B-cell non-Hodgkin's lymphoma characterized by the t(11;14)(q13,q32) translocation and over-expression of the cell cycle regulator protein, cyclin D1 [1]. The majority of patients present with advanced Ann Arbor stage III or IV, accompanied by peripheral blood and/or bone marrow involvement at diagnosis [2].
The clinical outcomes of MCL are highly heterogeneous, but, in general, MCL is considered an aggressive neoplasm; it has the poorest long-term survival rate of several mature B-cell lymphoma subtypes including marginal zone lymphoma, follicular lymphoma, and diffuse large B cell lymphoma [3]. Despite the development of treatment strategies, such as anti-CD20 monoclonal antibody therapy and hematopoietic stem cell transplantation, the prognosis for patients with MCL remains poor [2]. The median overall survival is 3–4 years [45].
Previous studies have reported that a high Ki-67 proliferation index (PI) in neoplastic cells is associated with poor survival in patients with MCL [6789]. A number of studies have proposed a particular Ki-67 PI cut-off value as an independent prognostic marker in MCL. However, those studies did not investigate this cut-off in terms of bone marrow involvement of MCL [6789]. Since MCL frequently presents with bone marrow involvement at diagnosis, it is necessary to also evaluate the clinical outcome according to the bone marrow findings.
In the present study, we aimed to determine the cut-off values of the Ki-67 PI as a prognostic factor in patients with MCL based on bone marrow findings.
We retrospectively reviewed the electronic medical records of patients who were diagnosed with MCL between 1995 and 2012 at Asan Medcal Center. The records of 89 patients with MCL were reviewed: 56 were included in the final analysis, and 33 were excluded due to the absence of Ki-67 PI index data for the tissue biopsies. Diagnoses of MCL were confirmed by pathological examination of lymph node biopsies. All 56 patients were treated with conventional combination chemotherapy as follows:CHOP [cyclophosphamide, doxorubicin, vincristine, prednisolone]; R-CHOP [rituximab-CHOP]; ESHAP [etoposide, methylprednisolone, cytarabine, cisplatin]; and Hyper-CVAD [Hyper-cyclophosphamide, -vincristine, -doxorubicin, -dexamethasone, plus methotrexate and cytarabine].
Immunohistochemical (IHC) staining for Ki-67 (Zymed Laboratories Inc., San Francisco, CA, USA) was performed on formalin-fixed paraffin-embedded tissue biopsy samples (N=56). The Ki-67 PI was calculated as the percentage of Ki-67-positive tumor cells identified in consecutive IHC staining assays for cyclin D1 in a representative area of the tissue biopsy specimen. Two experienced expert pathologists estimated the Ki-67 PI independently. If there was a difference in opinion, the Ki-67 PI was determined after discussion. There were no significant differences in the interpretation of the Ki-67 PI between the two pathologists. In patients with leukemic manifestation of MCL, additional Ki-67 IHC staining using bone marrow specimens (N=16) was performed.
To determine the cut-off value for the Ki-67 PI index, patients were grouped based on Ki-67 PI values (cut-off points: 10%, 20%, 30%, and 40%) and the associated survival rates were analyzed. The prognostic relevance of several factors, including age, gender, white blood cell (WBC) count, lactate dehydrogenase (LDH) level, and bone marrow findings, was analyzed using Cox regression analysis. The cut-off values for the various laboratory tests were determined using the reference values of the author's institution (WBC count, 10,000 cells/mm3; LDH, 250 IU/L). Clinical outcomes were compared using relative hazard ratios. Overall survival was calculated from the day of the initial diagnosis to death from any other cause or the last follow-up date. The chisquare test or Fisher's exact test was used for analysis of categorical data. The Mann-Whitney U test was used for analysis of continuous data. The Kaplan-Meier method and the log-rank test were used for survival curve analysis. Patients who had bone marrow involvement of MCL were analyzed separately.
Potential Ki-67 IHC staining correlations between the tissue and bone marrow biopsies were analyzed using Spearman's rank correlation analysis. Clinical data were analyzed using SPSS for Windows version 13.0 (SPSS, Chicago, IL). P<0.05 was considered statistically significant.
Patient demographics are summarized in Table 1. The median patient age was 63 years (range, 37–82 yr) and the male to female ratio was 2.3:1. The median follow-up period was 12.7 months (range, 0.4–76.2 mo).
Thirty-nine (69.6%) of the 56 patients showed bone marrow involvement of MCL, and 21 of these patients had leukemic manifestation at the time of diagnosis. The results of the Ki-67 IHC staining were as follows: <10% for 22 patients, 11–20% for 14 patients, 21–30% for 3 patients, 31–40% for 4 patients, 41–50% for 4 patients, and >50% for 9 patients. The IHC staining results for cyclin D1 were positive in 50 (89.3%) patients. Conventional cytogenetic analyses, performed in 51 patients, revealed a chromosomal abnormality in 6 (10.7%) patients who displayed t(11;14)(q13;q32). In total, 4 of the 56 patients showed a complex karyotype without t(11;14)(q13;q32).
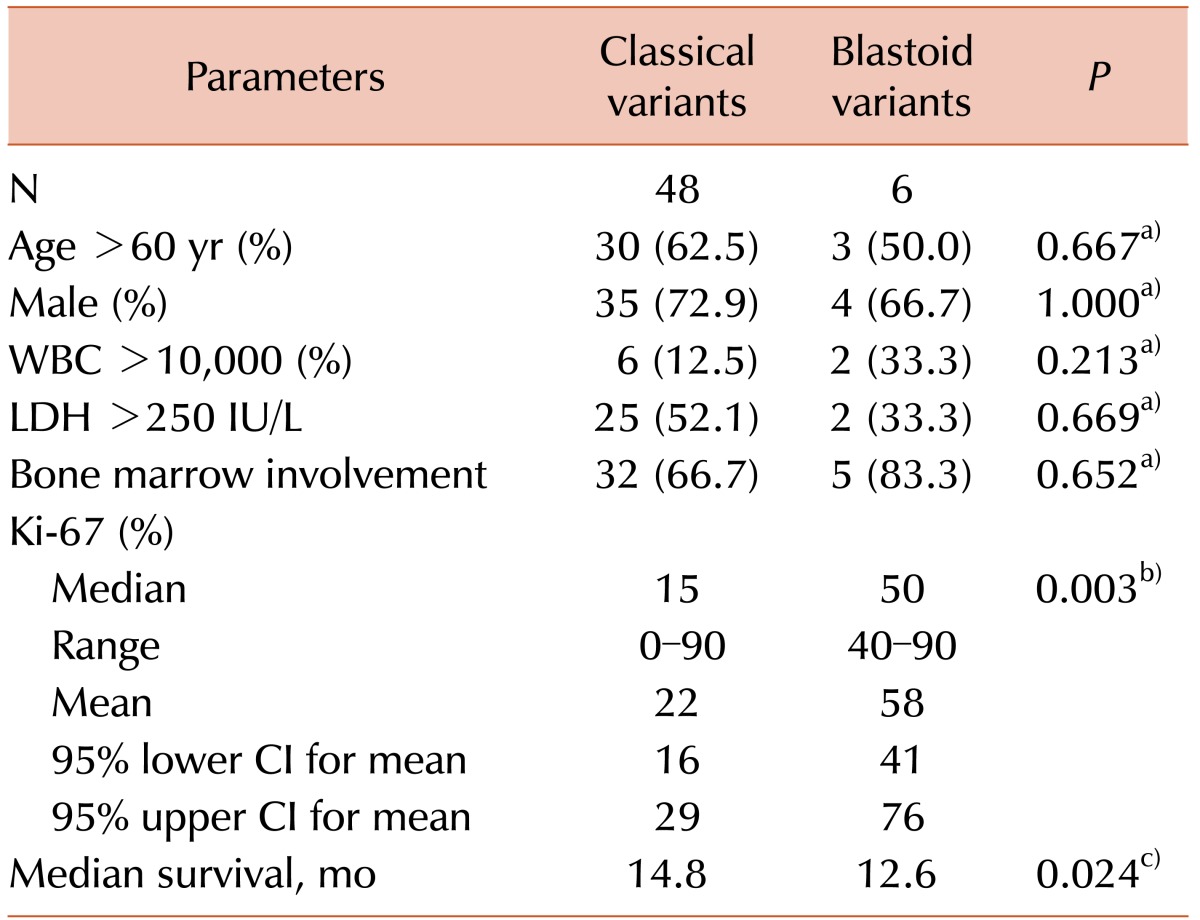
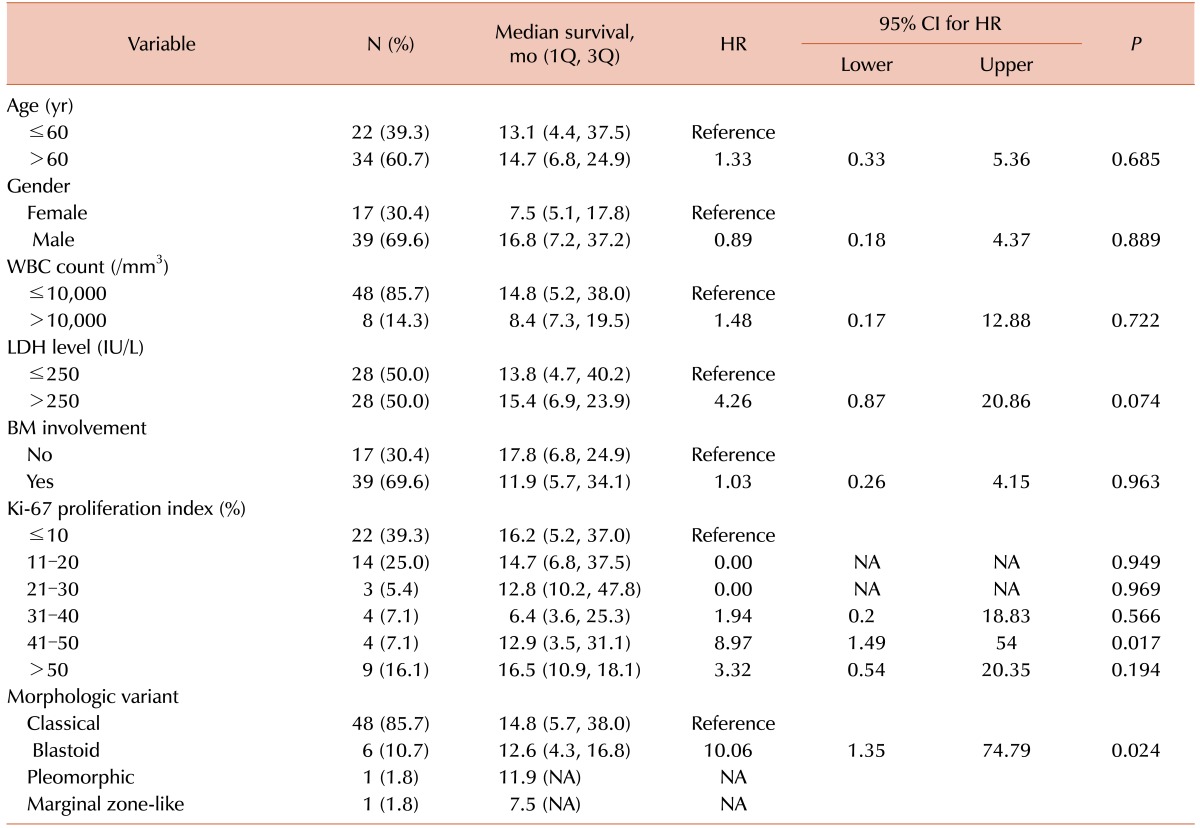
There was no association between clinical outcomes and age, gender, WBC count, LDH level, or bone marrow findings of MCL (Table 2). However, we observed a significant difference in overall survival according to the Ki-67 PI. Specifically, a high Ki-67 PI was associated with poor prognosis. Additionally, blastoid variants of MCL displayed a higher median Ki-67 PI (50% vs. 15%, P=0.003) and were associated with a poorer prognosis (15.6 mo vs. 64.9 mo, P=0.006) than classical MCL.
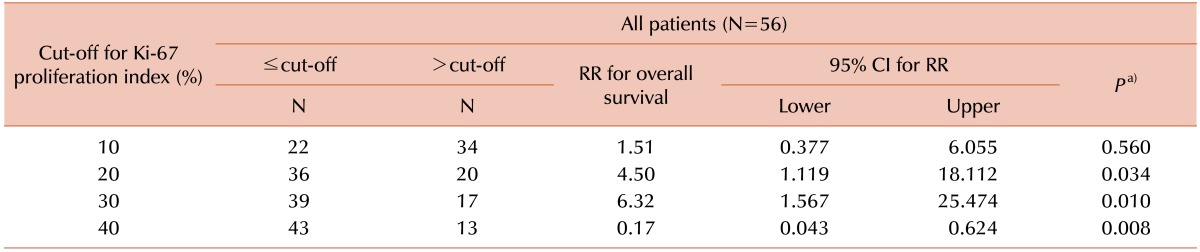
To determine the prognostic cut-off point for the Ki-67 PI, patients were divided into 2 groups according to Ki-67 PI cut-off values (Tables 3 and 4). At a cut-off value of 20%, significantly different survival curves with mean survival times of 69.8 months (Ki-67 PI≤20%) and 47.9 months (Ki-67 PI>20%) were obtained, regardless of bone marrow findings (P=0.034, Fig. 1A). However, for patients with bone marrow involvement of MCL, the statistically significant Ki-67 PI cut-off level increased to 30% (P=0.033, Fig. 1B).
An additional 16 bone marrow specimens were subjected to IHC. The IHC results revealed a positive correlation between Ki-67 staining patterns and tissue biopsy findings (Spearman's rank correlation coefficient of 0.559, P=0.024, Fig. 2). In particular, a good correlation was observed between a tissue biopsy Ki-67 PI <30% and the bone marrow biopsy results.
MCL is associated with rapid progression and high recurrence rates, resulting in poor long-term outcomes [1]. Several attempts have been made to subdivide patients into different risk groups; distinct results have been obtained with various factors, including age, gender, B symptoms, splenomegaly, hemoglobin levels, WBC counts, and LDH levels [16101112]. In the present study, age, gender, WBC count, LDH level, and bone marrow involvement of MCL were not associated with prognosis of MCL. However, the blastoid variant of MCL was associated with significantly poorer clinical outcomes than the classical variant. This is consistent with previous studies that showed that overall survival rates decreased in cases with prominent blastoid morphologies [613].
The most well-defined prognostic factors are the PI and the MCL International Prognostic Index (MIPI) [14]. In addition, a simplified MIPI and a biological MIPI have been reported [15]. Some researchers have suggested that the MIPI and the simplified MIPI may be associated with prognosis in MCL [1415], whereas others have reported no differences in survival after R-Hyper-CVAD therapy [16]. In the present study, Eastern Cooperative Oncology Group (ECOG) performance status data was not available for many patients at the time of the initial MCL diagnosis. Therefore, survival analysis according to the MIPI could not be performed.
To date, several studies have reported an association between a high Ki-67 PI and poor prognosis in MCL; in these studies, the cut-off values for the Ki-67 PI varied from 10% to 60% [67917181920]. The significance of the Ki-67 PI as a prognostic predictor in patients with MCL was investigated in a systematic meta-analysis study [21]. Combining the Ki-67 PI and the MIPI provided additional discriminatory power in the risk stratification of patients with MCL [1522]. Recently, a large cohort study from the European MCL Network also found that the Ki-67 PI was an independent prognostic factor in patients with MCL, and that it was superior to cytology and growth patterns [23]. Our results showed that a cut-off value of 20% for the Ki-67 PI was clinically meaningful, irrespective of bone marrow involvement in MCL. In a separate analysis in patients with bone marrow involvement, the statistically significant cut-off value increased to 30%. This increase in the cut-off value may be related to the more aggressive treatment administered to patients with bone marrow involvement, but further investigation is needed to confirm this finding.
In clinical laboratories, the manual cell counting method is generally used to interpret Ki-67 IHC results. Direct counting of 1,000 lymphoma cells has been considered the gold standard for assessing the Ki-67 PI in prospective randomized trials carried out by the European MCL Network [24]. However, this method of determination is subjective, and inter-observer variations are possible in the interpretation of data [25]. Recently, a more objective measurement technique for the Ki-67 PI was introduced. Schafell et al. used an image analysis system to interpret the Ki-67 IHC staining, thereby reducing inter-observer variation [25]. Brizova et al. [26] proposed the precise quantitative measurement of mRNA using real-time reverse transcription-polymerase chain reaction to determine the Ki-67 PI. However, these methods are not yet available in most clinical laboratories, and more objective measurement techniques for determination of the Ki-67 PI are necessary to effectively predict the prognosis of MCL.
Additional IHC staining for Ki-67 on bone marrow specimens revealed a significant positive correlation with the tissue biopsy results. Traditionally, lymphoma is diagnosed on the basis of excision or needle biopsy of tissue from affected lymph nodes or organs. However, in clinical practice, if a tissue biopsy is not possible, a bone marrow study is helpful for the diagnosis of lymphoma. In our study, IHC staining for Ki-67 in a representative area on bone marrow specimens was sufficiently informative to determine prognosis in leukemic manifestations of MCL. In particular, a good correlation with the results of the bone marrow biopsy was observed in tissue with a Ki-67 PI <30%. Therefore, Ki-67 IHC staining of bone marrow specimens may be employed to predict prognosis if a tissue biopsy is not available.
There are several limitations in this study. The small number of patients and the heterogeneous chemotherapy regimens, especially the inclusion or exclusion of rituximab, are major limitations in assessing the prognostic relevance of the Ki-67 PI in patients with MCL. In addition, the ECOG performance status which is a potential clinical prognostic indicator, as well as a component of the MIPI, could not be evaluated due to insufficient data. Large-scale and well-designed studies are needed to further elucidate the prognostic impact of the Ki-67 PI in patients with MCL.
In conclusion, the Ki-67 PI plays an important role in patients with MCL. Our results indicate that a 20% cut-off for the Ki-67 PI is clinically meaningful, regardless of bone marrow involvement of MCL. In addition, the statistically significant cut-off value increases to 30% in patients with bone marrow involvement of MCL.
References
1. Cortelazzo S, Ponzoni M, Ferreri AJ, Dreyling M. Mantle cell lymphoma. Crit Rev Oncol Hematol. 2012; 82:78–101. PMID: 21658968.

2. Lenz G, Dreyling M, Hiddemann W. Mantle cell lymphoma: established therapeutic options and future directions. Ann Hematol. 2004; 83:71–77. PMID: 14669040.

3. Ghielmini M, Zucca E. How I treat mantle cell lymphoma. Blood. 2009; 114:1469–1476. PMID: 19556426.

4. Jares P, Campo E. Advances in the understanding of mantle cell lymphoma. Br J Haematol. 2008; 142:149–165. PMID: 18410453.

5. Swerdlow SH, Williams ME. From centrocytic to mantle cell lymphoma: a clinicopathologic and molecular review of 3 decades. Hum Pathol. 2002; 33:7–20. PMID: 11823969.

6. Räty R, Franssila K, Joensuu H, Teerenhovi L, Elonen E. Ki-67 expression level, histological subtype, and the International Prognostic Index as outcome predictors in mantle cell lymphoma. Eur J Haematol. 2002; 69:11–20. PMID: 12270057.

7. Katzenberger T, Petzoldt C, Höller S, et al. The Ki67 proliferation index is a quantitative indicator of clinical risk in mantle cell lymphoma. Blood. 2006; 107:3407. PMID: 16597597.

8. Ek S, Björck E, Porwit-MacDonald A, Nordenskjöld M, Borrebaeck CA. Increased expression of Ki-67 in mantle cell lymphoma is associated with de-regulation of several cell cycle regulatory components, as identified by global gene expression analysis. Haematologica. 2004; 89:686–695. PMID: 15194536.
9. Determann O, Hoster E, Ott G, et al. Ki-67 predicts outcome in advanced-stage mantle cell lymphoma patients treated with anti-CD20 immunochemotherapy: results from randomized trials of the European MCL Network and the German Low Grade Lymphoma Study Group. Blood. 2008; 111:2385–2387. PMID: 18077791.

11. Vandenberghe E, De Wolf-Peeters C, Vaughan Hudson G, et al. The clinical outcome of 65 cases of mantle cell lymphoma initially treated with non-intensive therapy by the British National Lymphoma Investigation Group. Br J Haematol. 1997; 99:842–847. PMID: 9432032.

12. Argatoff LH, Connors JM, Klasa RJ, Horsman DE, Gascoyne RD. Mantle cell lymphoma: a clinicopathologic study of 80 cases. Blood. 1997; 89:2067–2078. PMID: 9058729.

13. Espinet B, Salaverria I, Beà S, et al. Incidence and prognostic impact of secondary cytogenetic aberrations in a series of 145 patients with mantle cell lymphoma. Genes Chromosomes Cancer. 2010; 49:439–451. PMID: 20143418.

14. Hoster E, Dreyling M, Klapper W, et al. A new prognostic index (MIPI) for patients with advanced-stage mantle cell lymphoma. Blood. 2008; 111:558–565. PMID: 17962512.
15. Geisler CH, Kolstad A, Laurell A, et al. The Mantle Cell Lymphoma International Prognostic Index (MIPI) is superior to the International Prognostic Index (IPI) in predicting survival following intensive first-line immunochemotherapy and autologous stem cell transplantation (ASCT). Blood. 2010; 115:1530–1533. PMID: 20032504.

16. Shah JJ, Fayad L, Romaguera J. Mantle Cell International Prognostic Index (MIPI) not prognostic after R-hyper-CVAD. Blood. 2008; 112:2583–2584. PMID: 18779406.

17. Tiemann M, Schrader C, Klapper W, et al. Histopathology, cell proliferation indices and clinical outcome in 304 patients with mantle cell lymphoma (MCL): a clinicopathological study from the European MCL Network. Br J Haematol. 2005; 131:29–38. PMID: 16173960.

18. Garcia M, Romaguera JE, Inamdar KV, Rassidakis GZ, Medeiros LJ. Proliferation predicts failure-free survival in mantle cell lymphoma patients treated with rituximab plus hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone alternating with rituximab plus high-dose methotrexate and cytarabine. Cancer. 2009; 115:1041–1048. PMID: 19170236.

19. Hsi ED, Jung SH, Lai R, et al. Ki67 and PIM1 expression predict outcome in mantle cell lymphoma treated with high dose therapy, stem cell transplantation and rituximab: a Cancer and Leukemia Group B 59909 correlative science study. Leuk Lymphoma. 2008; 49:2081–2090. PMID: 19021050.

20. Pervez S, Haroon S, Awan D. Ki-67 labeling indices in 'classic' versus 'blastoid' mantle cell lymphomas--proposed cutoff values for routine diagnostic workup. Asian Pac J Cancer Prev. 2015; 16:6591–6594. PMID: 26434879.
21. He X, Chen Z, Fu T, et al. Ki-67 is a valuable prognostic predictor of lymphoma but its utility varies in lymphoma subtypes: evidence from a systematic meta-analysis. BMC Cancer. 2014; 14:153. PMID: 24597851.

22. Vose JM. Mantle cell lymphoma: 2015 update on diagnosis, risk-stratification, and clinical management. Am J Hematol. 2015; 90:739–745. PMID: 26103436.

23. Hoster E, Rosenwald A, Berger F, et al. Tumor Cell Proliferation (Ki-67 Index) Overcomes Cytology and Growth Pattern As Prognostic Factor in Mantle-Cell Lymphoma – Results from Randomized Trials of the European MCL Network. Blood;2014. Accessed February 23, 2016. at http://www.bloodjournal.org/content/124/21/2977.
24. Klapper W, Hoster E, Determann O, et al. Ki-67 as a prognostic marker in mantle cell lymphoma-consensus guidelines of the pathology panel of the European MCL Network. J Hematop. 2009; 2:103–111. PMID: 19669190.

25. Schaffel R, Hedvat CV, Teruya-Feldstein J, et al. Prognostic impact of proliferative index determined by quantitative image analysis and the International Prognostic Index in patients with mantle cell lymphoma. Ann Oncol. 2010; 21:133–139. PMID: 20019090.

26. Brizova H, Kalinova M, Krskova L, Mrhalova M, Kodet R. A novel quantitative PCR of proliferation markers (Ki-67, topoisomerase IIalpha, and TPX2): an immunohistochemical correlation, testing, and optimizing for mantle cell lymphoma. Virchows Arch. 2010; 456:671–679. PMID: 20419314.
Fig. 1
Overall survival according to the Ki-67 proliferation index (PI). (A) A cut-off of 20% reveals significant differences in survival, regardless of bone marrow findings (N=56, P=0.034). (B) In patients with bone marrow involvement of mantle cell lymphoma, a cut-off of 30% is statistically significant (N=39, P=0.033).
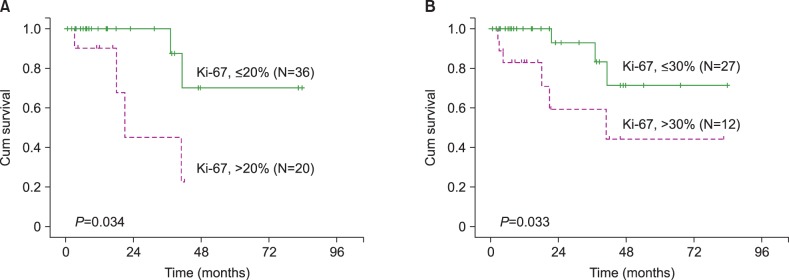
Fig. 2
Comparison of Ki-67 immunohistochemical staining results between tissue and bone marrow biopsies. A good correlation is observed between tissue biopsy with a Ki-67 PI <30% and bone marrow biopsy results (Spearman's correlation coefficient=0.559, P=0.024).

Table 2
Clinical outcomes of 54 patients with mantle cell lymphoma according to cytomorphological variant.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download