This article has been
cited by other articles in ScienceCentral.
Abstract
Background
Bloodstream infections (BSI) remain a frequent complication during the pre-engraftment period after hematopoietic stem cell transplantation (HSCT), resulting in high mortality rates. This study evaluated risk factors for mortality in hematopoietic stem cell transplant recipients with BSI in the pre-engraftment period.
Methods
This prospective case control study was performed at the Center of Hematology and Bone Marrow Transplantation in Minsk, Republic of Belarus. Data relating to patient age and gender, date and type of transplantation, conditioning chemotherapy regimen, microorganisms isolated from blood, and antibacterial therapy were prospectively collected from all hematopoietic stem cell recipients with microbiologically proven cases of BSI in the pre-engraftment period. The primary outcome was all-cause 30-day mortality after onset of febrile neutropenia.
Results
A total of 135 adult patients with microbiologically proven BSI after HSCT were studied, with 65.2% of cases caused by gram-negative microorganisms and 21.5% by non-fermenting bacteria. Inadequate empiric antibacterial therapy and isolation of carbapenem-resistant non-fermenting gram-negative bacteria (Acinetobacter baumannii and Pseudomonas aeruginosa) were independently associated with increased all-cause 30-day mortality in these patients.
Conclusion
The risk factors for mortality in adult patients with BSI in the pre-engraftment period after HSCT were inadequacy of empirical antibacterial therapy and isolation of carbapenem-resistant A. baumannii or P. aeruginosa.
Go to :

Keywords: Hematopoietic stem cell transplantation, Bloodstream infection, Risk factors, Antibacterial therapy
INTRODUCTION
Bloodstream infections (BSIs) remain an important cause of morbidity and mortality in recipients of hematopoietic stem cell transplantation (HSCT). BSIs occur in 13.0–55.8% of HSCT recipients, with mortality rates ranging from 24.0% to 43.6% [
123456]. Although previously published studies provide some important information, the modern challenges of the emergence of highly resistant gram-negative pathogens requires new information about the risk factors and etiological spectrum of BSI in HSCT recipients. Knowledge of risk factors for mortality in these patients is key for choosing appropriate treatment regimens. Lack of published data about the causes of BSI in patients receiving HSCT and poor knowledge of risk factors for adverse outcomes in such patients may contribute to inadequate empirical antibacterial therapy. The current study assessed risk factors for mortality and modern causes of BSI in HSCT recipients in the pre-engraftment period.
Go to :

MATERIALS AND METHODS
Setting and design
The Republican Center for Hematology and Bone Marrow Transplantation is a national clinical and research center for adult patients located in Minsk, Republic of Belarus. The clinical departments are based in the 9th Clinical Hospital of Minsk, which is one of the largest teaching hospitals in Belarus, performing more than 100 HSCTs annually. The Center has 150 beds, including an intensive care unit, for patients with various hematological diseases and patients undergoing HSCT. The Center also includes laboratories for microbiology, bone marrow separation and freezing, cellular biotechnology, human leukocyte antigen (HLA)-typing, and clinical diagnostics.
A prospective observational study was performed to determine the possible risk factors for adverse outcome in adult patients with microbiologically proven BSI in the pre-engraftment period after HSCT. The indications for HSCT included acute myeloid leukemia, chronic myeloid leukemia, acute lymphoblastic leukemia, chronic lymphocytic leukemia, myelodysplastic syndromes, multiple myeloma, Hodgkin's lymphoma, and non-Hodgkin's lymphoma. The study was approved by the Scientific and Ethical Committees of the Republican Center for Hematology and Bone Marrow Transplantation in Minsk, Republic of Belarus.
The primary outcome in this study was all-cause 30-day mortality after onset of febrile neutropenia. The covariates included in analysis were: patient age and gender characteristics, type of HSCT (autologous/allogeneic), conditioning chemotherapy regimen (myeloablative/non-myeloablative or reduced intensity), primary diagnosis, level of neutropenia on the day that the first positive blood culture was collected, isolation of carbapenem-resistant non-fermenting gram-negative bacteria (Acinetobacter baumannii and Pseudomonas aeruginosa), isolation of extended-spectrum beta-lactamases (ESBL)-producing member of Enterobacteriaceae spp. family, isolation of methicillin-resistant S. aureus (MRSA), and adequacy of empirical antibacterial therapy.
Data collection
Epidemiological, clinical, and laboratory data were prospectively collected for patients aged 18–70 years undergoing HSCT from January 2013 to October 2015. The exclusion criteria included concurrent active oncological disease, hepatitis B or hepatitis C infection, active fungal disease, rheumatological diseases, and diabetes mellitus. Patients with possible active cytomegalovirus (CMV) infection (monitored using real-time quantitative polymerase chain reaction) were also excluded from the study. All patients had a complete clinical and hematological remission of the main disease at the start of HSCT. Blood cultures were obtained from all patients with standard precautions and fulfilled the criteria of febrile neutropenia in the pre-engraftment period after HSCT, with identification and in vitro antibiotic susceptibility testing. Every patient with BSI was followed for at least 30 days after collection of the first positive blood culture. Only first bacteremia episodes were included in the analyses.
Definitions
Empirical antimicrobial therapy was defined as adequate if it was administered <24 hours after collection of blood cultures and if the microorganisms subsequently isolated
in vitro were susceptible to at least one of the administered antibiotics. Empirical antimicrobial therapy was defined as inadequate if the subsequently isolated microorganisms were resistant or intermediately susceptible
in vitro to all of the administered antibiotics, or the empiric antibacterial therapy was administered >24 hours after collection of blood cultures or the dosing regimen conflicted with the standard dosing recommendations. The 30-day mortality was defined as the number of patients with BSI who died within 30 days after the onset of febrile neutropenia divided by the total number of patients with BSI. Adverse outcome was defined as death within 30 days from the onset of febrile neutropenia. The pre-engraftment period after HSCT was defined as the period from day 0 to day 30 after HSCT [
7]. BSI was defined as having a microbiologically proven growth from a blood culture after HSCT in patients with febrile neutropenia. The criteria for febrile neutropenia included a single oral temperature measurement >38.3℃ or a temperature >38.0℃ sustained over a 1-h period in a patient with absolute neutrophil count (ANC) of <500 cells/µL or an ANC expected to decrease to <500 cells/µL during the next 48 hours [
8].
Transplantation procedure and infection management
Transplantations were performed according to institutional protocols. Briefly, the most frequent myeloablative conditioning regimens were busulfan plus cyclophosphamide or cyclophosphamide plus total-body irradiation. Non-myeloablative and reduced-intensity conditioning mainly included fludarabine with melphalan or treosulfan and BEAM regimen (carmustine, etoposide, cytarabine, melphalan). Graft-versus-host disease (GVHD) prophylaxis regimens included cyclosporine, methotrexate, and tacrolimus. Antithymocyte globulin was administered in cases of unrelated donors. Standard antibacterial prophylaxis in the department was based on fluoroquinolones (mainly ciprofloxacin 0.5 g BID orally) starting from the initiation of the conditioning regimen until the level of neutrophils in the peripheral blood exceeded 500 cells/µL. No routine antibacterial prophylaxis against Streptococcus pneumoniae was administered. Antifungal prophylaxis with fluconazole was prescribed to patients undergoing autologous HSCT, while micafungin was used as antifungal prophylaxis in patients undergoing allogeneic HSCT. Prophylaxis against Pneumocystis jirovecii with trimethoprim-sulfamethoxazole was administered to all patients until immunologic recovery after HSCT. Acyclovir was used as prophylaxis for infections caused by herpes viruses. Real-time quantitative polymerase chain reaction was used to monitor CMV DNA levels in HSCT patients weekly during the pre-engraftment period with ganciclovir as the first-line pre-emptive therapy in case of possible active CMV infection and exclusion of such patients from the study. During the period of severe neutropenia (ANC <100 cells/µL), all patients were isolated in single rooms with positive pressure, laminar air flow, and high-efficiency particulate air filtration. After the ANC exceeded 100 cells/µL, some of the clinically stable patients were moved to the intensive care department with two patients per room and positive air pressure.
The institution's standard protocols for initial empirical antibiotic therapy for treatment of febrile neutropenia included cephalosporins (cefepime or cefoperazone/sulbactam) or carbapenems (imipenem/cilastatin or meropenem) depending on the patient risk group, with an addition of vancomycin in cases of possible infection caused by gram-positive pathogens [
9].
Blood cultures
In every patient with febrile neutropenia before administration of antibacterial therapy, 10 mL of blood was taken both from the peripheral vein and the central venous catheter with standard aseptic precautions and cultivated in aerobic/anaerobic bioMerieux BacT/ALERT culture media in a BacT/ALERT 3D automated microbial detection system until receiving positive results or until the seventh day. In cases of positive results, the microbial culture was isolated and grown on different manufactured culture media. Identification and antimicrobial susceptibility testing were performed using a bioMerieux VITEK 2 automatic system, and the ESBL phenotype was determined using a VITEK 2 ESBL Test System. Additional antimicrobial susceptibility in carbapenem-resistant strains (resistance to imipenem, meropenem, and doripenem) was confirmed by E-tests and disc-diffusion assays. The minimum inhibitory concentration (MIC) breakpoints used for susceptibility testing were most current Clinical and Laboratory Standards Institute (CLSI) guideline at the start of the study [
10]. The criteria for establishing a bacterial isolate as clinical significant included clinical signs of active infection or isolation of the same microorganism with identical antimicrobial resistance profiles from more than one blood culture bottle or more than once during a one-week period.
Statistical analysis
Non-parametric statistics for categorical (Chi-squared or Fisher's exact tests) and quantitative (Mann-Whitney U-test, Odds Ratio) variables were used for statistical analysis. The distributions of the variables were determined using the Shapiro-Wilk test. Multivariate analysis was performed using logistic regression methods for categorical variables with P≤0.2 in univariate analysis. Data processing and analysis was performed using MedCalc Statistical Software v.14.10.2 (MedCalc Software bvba, Ostend, Belgium), and results were considered statistically significant for P<0.05.
Go to :

RESULTS
During the study period, a total 360 transplantations were performed at our center, of which 62 and 298 were allogeneic and autologous, respectively. Positive blood culture results were obtained in 135 patients, leading to an overall BSI incidence of 37.5%. The 30-day mortality in patients with BSI in the pre-engraftment period after HSCT was 31.1%. The median day of the first febrile episode with subsequently confirmed BSI (N=135) was day 5 (interquartile range, 4–7 d) following HSCT. The baseline demographic and clinical characteristics of the patients included in the study are shown in
Table 1. The most significant characteristics of patients associated with 30-day mortality in univariate analysis (
Table 2). Risk factors that showed statistical significance in univariate analysis were subsequently checked for independency in multivariate analysis performed by logistic regression.
Table 1
Demographic and clinical baseline characteristics of patients with BSI in the pre-engraftment period after HSCT (N=135).
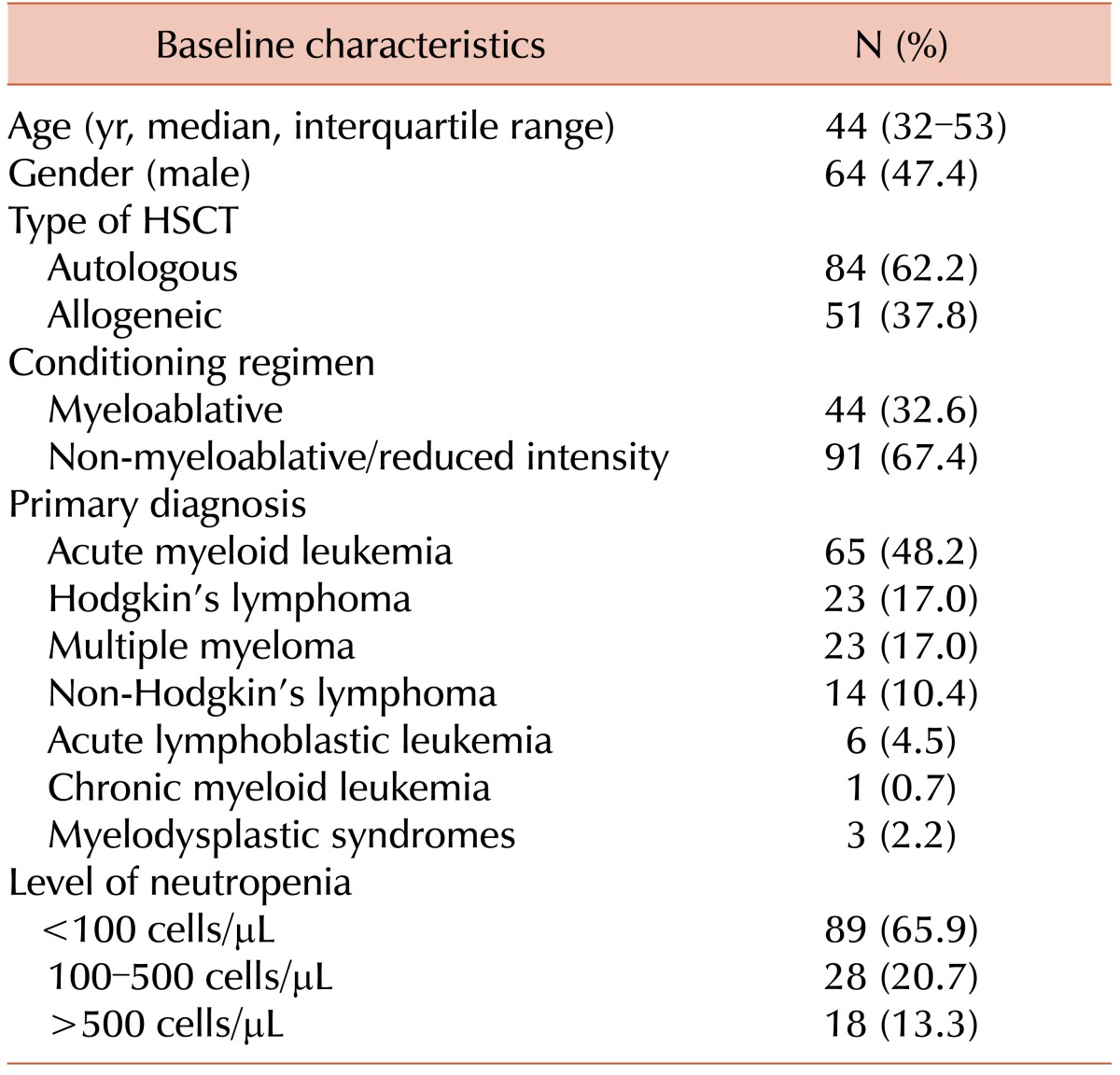

Table 2
Risk factors for mortality in adult patients with BSI in pre-engraftment period after HSCT in univariate analysis.

The disease stage was not included in the analysis since all the patients had complete remission of their primary disease at the start of HSCT. Therefore, the statistically significant risk factors for 30-day mortality in adult patients with BSI who had received HSCT in univariate analysis included inadequate empirical antibacterial therapy (OR 20.82; 95% CI 8.06–53.78;
P<0.0001) and isolation of carbapenem-resistant
A. baumannii or
P. aeruginosa (OR 7.37; 95% CI 2.54–21.35; P =0.0002). The group of patients with acute myeloid leukemia was also at higher risk of adverse outcome (OR 3.03; 95% CI 1.41–6.5;
P=0.0045). This group was further analyzed for possible confounding factors, and four patients were excluded from the multivariate analysis since autopsy data of these deceased patients to confirm minimal residual disease (MRD) were not available. Isolation of MRSA or ESBL-producers was not a risk factor for 30-day mortality in multivariate analysis. The results of the multivariate analysis indicate that infection caused by carbapenem-resistant
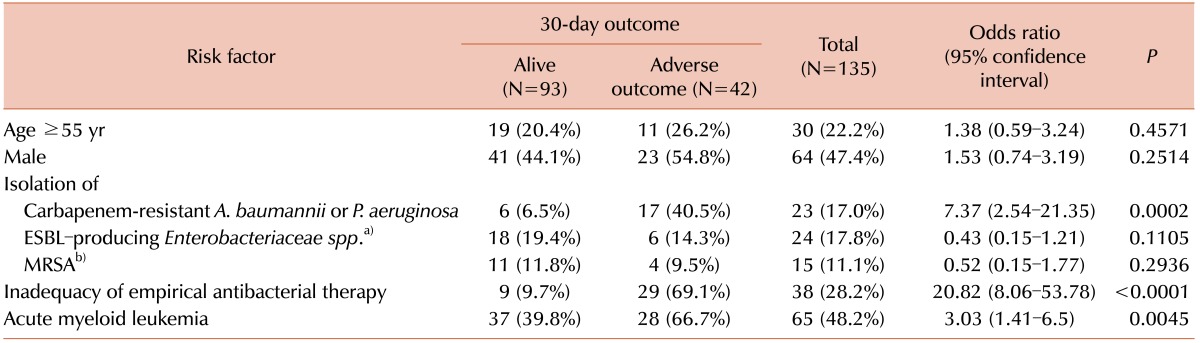
P. aeruginosa or
A. baumannii was an independent risk factor for 30-day mortality in patients with BSI in the pre-engraftment period after HSCT (regression coefficient 1.697; standard error 0.68;
P=0.0126). Inadequacy of empirical antibacterial therapy was also a statistically significant independent risk factor of 30-day mortality in these patients (regression coefficient 2.71; standard error 0.57;
P<0.0001). The results of multivariate analysis are shown in
Table 3.
Table 3
Results of multivariate analysis of risk factors for 30-day mortality in patients with BSI in the pre-engraftment period after HSCT.
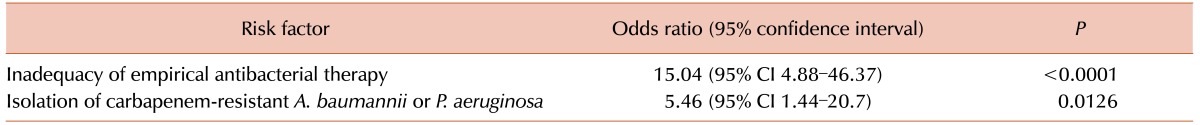

Microbiological data on the causes of BSI in the pre-engraftment period after HSCT indicated the major impact of gram-negative bacterial flora.
Table 4 shows the spectrum of BSI-causing bacteria. Among the identified causative pathogens of BSI in adult patients after HSCT, gram-negative microorganisms and non-fermenters (
A. baumannii,
P. aeruginosa, and
S. maltophilia) comprised 65.2% and 21.5%, respectively, of the etiological spectrum.
Table 4
Causes of BSI in the pre-engraftment period after HSCT.

Among the K. pneumonia isolates (N=34), 18 (52.9%) showed resistance to cephalosporins, while seven (20.6%) were resistant to carbapenems. Among Escherichia coli isolates (N=25), only six (24.0%) were resistant to cephalosporins, and no resistance to carbapenems was detected. The carbapenem-resistance among gram-negative non-fermenting bacteria (N=29) was as follow: 12 of 16 A. baumannii isolates, 10 of 12 P. aeruginosa isolates, and one S. maltophilia isolate. Among Staphylococcus aureus (N=23) isolates, 15 (65.2%) demonstrated a methicillin-resistant phenotype, for which anti-gram-positive empirical antibacterial coverage should be considered. Among 18 coagulase-negative Staphylococci isolates, only five (27.8%) were resistant to oxacillin. Only a few S. pneumoniae and Enterococcus spp. were isolated in the current study, which made it difficult to discuss their levels of antibiotic resistance in post-HSCT patients.
Go to :

DISCUSSION
Inadequate empirical antimicrobial therapy in intensive care units is associated with excess mortality [
11]. However, its clinical impact in patients with BSI in the pre-engraftment period after receiving HSCT remains controversial owing to the limited number of observations. The current study shows the clinical significance of adequate empiric antibacterial therapy in adult patients undergoing HSCT, with high 30-day mortality rates in patients with inappropriate antibiotic therapy for treatment of febrile neutropenia. This finding underscores the importance of the knowledge of the local spectrum of pathogens, which may be helpful when selecting the appropriate empiric antibacterial therapy regimen. The high rate of gram-negative pathogens in our study corresponds with a similar trend in several European countries [
912]. The high mortality rate in patients with BSI (31.1%) in this study and other similar studies showing mortality rates of 15.0–20.0% [
513] may be due to local outbreaks of metallo-beta-lactamase-producing
P. aeruginosa and
A. baumannii infections, which should be investigated further using molecular methods such as multilocus sequence typing. Carbapenem-resistant
A. baumannii or
P. aeruginosa was identified as a significant risk factor for 30-day mortality; isolation of these bacteria should be notified to clinicians to start an adequate dosing regimen with intense antibacterial therapy including colistin.
The main limitation of our study was the relatively small sample size (N=135); however, considering the cost of the HSCT procedure, even this limited number of observations may yield important information. The other limitation was that our definition of inadequate empirical antibacterial therapy, which was based on in vitro susceptibility. The antibiotic could still be clinically effective in vivo in some cases. In addition, it was not always possible to confirm if the main cause of mortality was BSI.
In conclusion, the risk factors for mortality in adult patients with BSI in the pre-engraftment period after HSCT were inadequacy of empirical antimicrobial therapy and isolation of carbapenem-resistant A. baumannii or P. aeruginosa.
Go to :






 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download