Abstract
Background
Few clinical studies have clarified the prognostic factors that affect clinical outcomes for patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) after immunochemotherapy.
Methods
A total of 158 patients with relapsed or refractory DLBCL were enrolled. All patients underwent positron emission tomography/computed tomography (PET/CT) before and after salvage therapy. All enrolled patients previously received the ifosfamide, carboplatin, and etoposide regimen. Clinical outcomes were compared according to several factors (age ≥ 65 years, low age-adjusted International Prognostic Index [aa-IPI], maximum standardized uptake value [SUVmax] <6.0 on PET/CT, time to relapse ≥12 months, complete response after salvage therapy). A low aa-IPI, SUVmax <6.0, and time to relapse ≥ 12 months were independent prognostic factors for survival.
Go to : 
The combination of rituximab with cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) has produced significant survival benefits, especially in elderly patients with untreated diffuse large B-cell lymphoma (DLBCL), compared to the CHOP regimen [1]. Although the immunochemotherapy improved clinical outcomes, some patients experience early refractoriness, unsatisfactory response, or relapse.
High-dose therapy (HDT) with autologous stem cell transplantation (ASCT) has become the standard of care for younger patients with chemosensitive relapsed non-Hodgkin lymphoma (NHL) and patients with primary refractory aggressive NHL [2]. In a recent study, the second-line age-adjusted International Prognostic Index (aa-IPI) accurately reflected prognosis in a group of patients with relapsed NHL [3]. The intergroup Collaborative Trial in Relapsed Aggressive Lymphoma evaluated salvage therapy regimens in HDT/ASCT-eligible patients, and the study identified several prognostic factors, including prior exposure to rituximab, early relapse (<12 months), and a secondary IPI of 2-3 [4]. In other studies, the use of 18-fluorine fluorodeoxyglucose (18F-FDG) positron emission tomography/computed tomography (PET/CT) in the relapsed setting was found to predict patient outcomes [56789].
Although HDT/ASCT is the standard treatment for improving clinical outcomes in relapsed patients, the treatment is associated with an extremely poor prognosis in elderly patients with relapsed or refractory DLBCL and for patients who are ineligible for HDT/ASCT. However, little is known regarding the prognostic factors that affect the clinical outcomes of patients.
The present study investigated the relationships and clinical value of several prognostic parameters and clinical outcomes in patients with relapsed or refractory DLBCL who are ineligible for HDT/ASCT.
Go to : 
A retrospective review of records was performed at five medical centers in Korea (Pusan National University Hospital, Chonnam National University Hwasun Hospital, Seoul National University Bundang Hospital, Gachon University Gil Medical Center, and Pusan National University Yangsan Hospital). Patients who experienced relapse following R-CHOP therapy for DLBCL between 2006 and 2012 were included. Patients were excluded from the study if they had received additional therapies such as rituximab maintenance therapy after R-CHOP or underwent ASCT. During the period, all patients received treatment with the ifosfamide, carboplatin, and etoposide (ICE) regimen. The patients who received radiotherapy, and other types of salvage chemotherapy were excluded. This study was approved by the local Institutional Review Board.
The ICE regimen was also administered in an inpatient setting. Ifosfamide was administered intravenously as a single dose of 5 g/m2/day on day 2 with mesna. Carboplatin was administered at a dose of 800 mg on day 2. Etoposide was administered intravenously at a dose of 100 mg/m2/day from day 1 to day 3. These two schedules were administered every 3 weeks.
For each patient, the aa-IPI was assessed using data obtained immediately before the initiation of salvage therapy. The aa-IPI factors consist of elevated serum lactate dehydrogenase (LDH) levels above the upper limit of the normal range, Ann Arbor stage III or IV, and Eastern Cooperative Oncology Group (ECOG) performance status (PS) above grade 2. In the present study, each group was classified according to the sum of these risk factors as follows: the low-risk group had no risk factors, the intermediate-risk group had one risk factor, and the high-risk group had two or three risk factors.
All patients underwent an initial 18F-FDG-PET/CT scan before and after salvage therapy. All of the scans were analyzed using fusion software (Syngo, Siemens Medical Solutions, Malvern, PA, USA). By using the software package, the scans were evaluated in trans-axial, sagittal, and coronal views. Target lesions with a largest diameter >1 cm were identified on baseline 18F-FDG PET/CT scans. The maximum standardized uptake value (SUVmax) of each lesion was measured using the initial PET/CT scan. For each patient, the lesions with the greatest SUVmax were considered representative.
After the diagnosis of relapse, the patients underwent a conventional re-staging work-up including a physical examination, complete blood cell counts and blood chemistry, CT (neck, chest, and abdominal-pelvic area), and PET/CT. The final response was assessed by using conventional CT and PET/CT. Bone marrow biopsy was performed only if it had been involved before salvage therapy. The response was assessed at 3 weeks after salvage therapy using the revised criteria of Cheson et al. [10].
Progression-free survival (PFS) was defined as the time between the beginning of salvage therapy until disease progression or death. Overall survival (OS) was defined as the time from the start of salvage therapy until death. The Kaplan-Meier method was used to estimate survival, and 95% confidence intervals (CIs) were calculated. Cox progression analysis was used to calculate the hazard ratio between the two arms. Receiver operating characteristic (ROC) curves were prepared to estimate the accuracy of predicting the ideal cut-off of SUVmax. The statistical analysis was conducted using SPSS software version 18.0 (SPSS Inc., Chicago, IL, USA). A P-value of <0.05 was considered significant.
Go to : 
The baseline characteristics of the patients are summarized in Table 1. All 158 patients received ICE therapy. The male-to-female ratio was 102:56. The median patient age was 66 years (range, 20-85 years). One hundred patients (63.2%) were older than 60 years. In total, 102 patients (64.5%) had advanced disease (including stage III and IV), and 72 patients (45.4%) had an ECOG PS above grade 2. Ninety-nine patients (62.6%) had B symptoms, and the LDH level was elevated in 94 patients (59.4%). Forty-two patients experienced relapses that occurred 12 months after R-CHOP therapy (26.5%). Twelve patients (7.5%) achieved complete responses (CRs) after salvage therapy. The median follow-up duration was 32.6 months. During a follow-up period of 36.8 months, the PFS and OS rates were 26.9% and 33.5%, respectively, in the ICE group. The median number of chemotherapy cycles was 4.
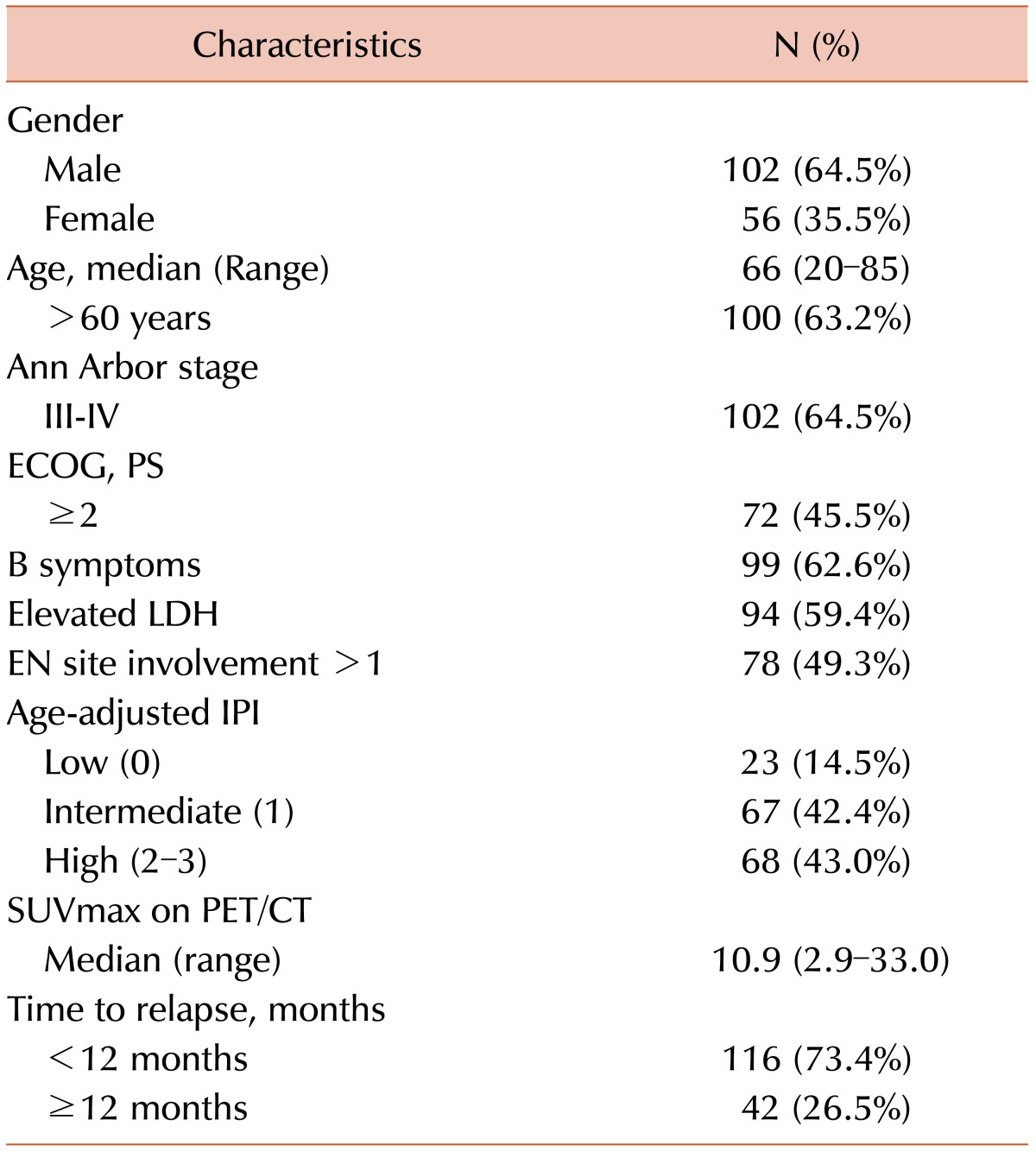
There were 23 patients in the low-risk group (0 aa-IPI risk factor). There were 67 patients in the intermediate risk group (1 risk factor), and 76 patients in the high-risk group (2-3 risk factors). Significant differences in PFS and OS were noted between the low- and high-risk groups (PFS, P <0.001 between the low- and high-risk groups; OS, P <0.001 between the low- and high-risk groups, Fig. 1A-B).
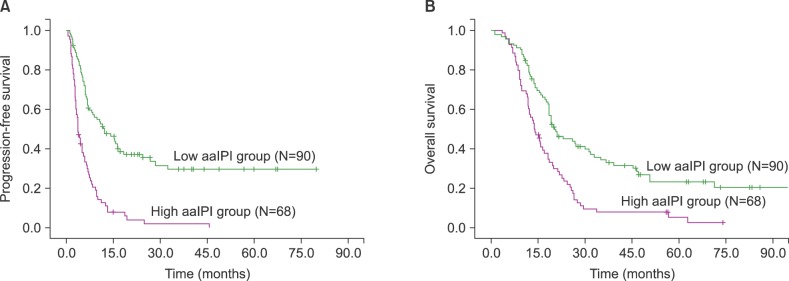
The median SUVmax was 10.9 (range, 2.9-33.0). The ideal cut-off for SUVmax was 6.0 according to ROC curve analysis. The area under the ROC curve of SUVmax was 0.811. The sensitivity and specificity were 90.1 and 73.1%, respectively. Depending on his or her SUVmax, each patient was categorized into either the low (<6.0) or high SUVmax group (≥6.0). According to this cut-off value, significant differences in survival were observed (P <0.001 for PFS and P <0.001 for OS, Fig. 2A-B).
To examine whether clinical outcomes were affected by various prognostic factors (age≥65 years, low aa-IPI, SUVmax <6.0, time-to-relapse >12 months, and CR status after salvage therapy), the factors were analyzed regarding their prognostic significance for salvage therapy.
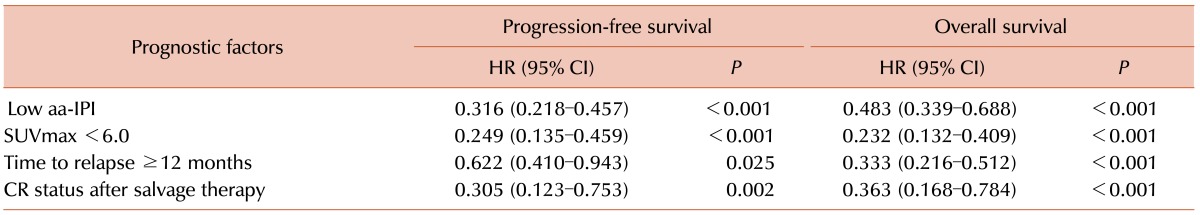
In univariate analysis, a low aa-IPI (P <0.001 for PFS, P <0.001 for OS), SUVmax <6.0 (P <0.001 for PFS, P <0.001 for OS), time to relapse >12 months (P =0.025 for PFS, P <0.001 for OS), and CR status after the therapy (P =0.002 for PFS, P <0.001 for OS) were associated with both PFS and OS (Table 2).
Multivariate analysis revealed that a low aa-IPI (P <0.001 for PFS, P <0.001 for OS), SUVmax <6.0 (P <0.001 for PFS, P <0.001 for OS), and time to relapse ≥12 months (P <0.057 for PFS, P <0.001 for OS) were independent prognostic factors associated with favorable outcomes (Table 3).
Go to : 
Few studies have been devoted to therapeutic options for patients with relapsed DLBCL who are ineligible for HDT/ASCT. It is well known that the prognosis is extremely poor with few therapeutic possibilities. The best therapeutic intervention is palliative chemotherapy together with other interventions to preserve a high quality of life. However, some subgroups of relapsed patients with low risk achieve a second good response and experience prolonged survival. Therefore, it is important to identify prognostic factors for predicting long-term outcomes in this population.
Several studies suggested the clinical value of certain prognostic factors including a tumor mass dimension larger than 10 cm, therapeutic regimens prior to ASCT, elevated LDH levels, short time to relapse, and an overall high disease burden [91011121314]. However, the widely accepted single prognostic model in the relapsed/refractory setting could not be generalized. A recent study illustrated the prognostic significance of aa-IPI based on the clinical data of patients with relapsed NHL who underwent HDT/ASCT [151617]. Guglielmi et al. also performed a retrospective analysis of aa-IPI factors for patients with relapsed DLBCL and identified relapse within 12 months of the initial diagnosis and aa-IPI factors as significant independent prognostic factors [18]. In addition, other clinical studies demonstrated the positive predictive value of aa-IPI [31920]. However, these results cannot be generalized to all patients with NHL because the aa-IPI factors were mostly focused on patients who underwent HDT/ASCT. Conversely, the present study was designed to evaluate the prognostic value of aa-IPI for patients with relapsed or refractory DLBCL who were ineligible for HDT/ASCT, and the data suggested that a low aa-IPI is associated with favorable outcomes for HDT/ASCT-ineligible patients.
Several research groups have investigated the use of 18F-FDG PET/CT to predict the outcomes of patients with relapsed or refractory NHL as well as newly diagnosed patients. In particular, it is well established that a CR on PET/CT or the negative conversion of FDG uptake after salvage therapy was associated with favorable outcomes [2122]. However, it is not known whether the degree of metabolic activity at the time of relapse influences clinical outcomes. In the present study, the clinical significance of the initial SUVmax and CR on PET/CT was evaluated in the relapsed setting. Interestingly, an initial high SUVmax at relapse was associated with an advanced Ann Arbor stage compared to a low SUVmax, and it had significant prognostic value for predicting poor clinical outcome. Meanwhile, the CR status did not have significance in the multivariate analysis. To confirm our result, additional clinical data are needed.
Recent studies demonstrated that SUVmax on pre-treatment 18F-FDG PET/CT was an important prognostic factor for patients with relapsed NHL who were treated with radioimmunotherapy [1011]. Salvage therapeutic regimens were used in patients with relapsed or refractory DLBCL. The present study only revealed clinical outcomes after ICE salvage therapeutic regimens. Therefore, further well-designed studies are needed to confirm our study.
In conclusion, the present study illustrated the prognostic significance of aa-IPI and SUVmax for patients with relapsed DLBCL who did not undergo ASCT. The aa-IPI and SUVmax were available to predict the survival outcomes in patients with relapse regardless of the use of salvage therapy. Although our study had limitations associated with its small sample size, the retrospective nature of its design, and the lack of rituximab assignment to salvage therapy, no prior study assessed the prognostic factors affecting patients with relapsed DLBCL who did not or could not undergo ASCT.
Go to : 
Notes
Authors' Disclosures of Potential Conflicts of Interest: No potential conflicts of interest relevant to this article were reported.
Go to : 
References
1. Coiffier B, Thieblemont C, Van Den Neste E, et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d'Etudes des Lymphomes de l'Adulte. Blood. 2010; 116:2040–2045. PMID: 20548096.

2. Philip T, Guglielmi C, Hagenbeek A, et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin's lymphoma. N Engl J Med. 1995; 333:1540–1545. PMID: 7477169.

3. Hamlin PA, Zelenetz AD, Kewalramani T, et al. Age-adjusted International Prognostic Index predicts autologous stem cell transplantation outcome for patients with relapsed or primary refractory diffuse large B-cell lymphoma. Blood. 2003; 102:1989–1996. PMID: 12676776.

4. Gisselbrecht C, Glass B, Mounier N, et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol. 2010; 28:4184–4190. PMID: 20660832.

5. Spaepen K, Stroobants S, Dupont P, et al. Prognostic value of pretransplantation positron emission tomography using fluorine 18-fluorodeoxyglucose in patients with aggressive lymphoma treated with high-dose chemotherapy and stem cell transplantation. Blood. 2003; 102:53–59. PMID: 12609836.

6. Schot BW, Zijlstra JM, Sluiter WJ, et al. Early FDG-PET assessment in combination with clinical risk scores determines prognosis in recurring lymphoma. Blood. 2007; 109:486–491. PMID: 17003382.

7. Dickinson M, Hoyt R, Roberts AW, et al. Improved survival for relapsed diffuse large B cell lymphoma is predicted by a negative pre-transplant FDG-PET scan following salvage chemotherapy. Br J Haematol. 2010; 150:39–45. PMID: 20507301.

8. Lim I, Park JY, Kang HJ, et al. Prognostic significance of pretreatment 18F-FDG PET/CT in patients with relapsed/refractory B-cell non-Hodgkin's lymphoma treated by radioimmunotherapy using 131I-rituximab. Acta Haematol. 2013; 130:74–82. PMID: 23548464.
9. Lopci E, Santi I, Derenzini E, et al. FDG-PET in the assessment of patients with follicular lymphoma treated by ibritumomab tiuxetan Y 90: multicentric study. Ann Oncol. 2010; 21:1877–1883. PMID: 20147744.

10. Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007; 25:579–586. PMID: 17242396.
11. Vose JM, Anderson JR, Kessinger A, et al. High-dose chemotherapy and autologous hematopoietic stem-cell transplantation for aggressive non-Hodgkin's lymphoma. J Clin Oncol. 1993; 11:1846–1851. PMID: 8105034.

12. Prince HM, Imrie K, Crump M, et al. The role of intensive therapy and autologous blood and marrow transplantation for chemotherapy-sensitive relapsed and primary refractory non-Hodgkin's lymphoma: identification of major prognostic groups. Br J Haematol. 1996; 92:880–889. PMID: 8616081.

13. Moskowitz CH, Bertino JR, Glassman JR, et al. Ifosfamide, carboplatin, and etoposide: a highly effective cytoreduction and peripheral-blood progenitor-cell mobilization regimen for transplant-eligible patients with non-Hodgkin's lymphoma. J Clin Oncol. 1999; 17:3776–3785. PMID: 10577849.

14. de Kreuk M, Ossenkoppele GJ, Meijer CJ, Huijgens PC. Prognostic factors for survival of non-Hodgkin's lymphoma patients treated with high-dose chemotherapy and autologous bone marrow transplantation. Bone Marrow Transplant. 1996; 17:963–971. PMID: 8807101.
15. Rapoport AP, Rowe JM, Kouides PA, et al. One hundred autotransplants for relapsed or refractory Hodgkin's disease and lymphoma: value of pretransplant disease status for predicting outcome. J Clin Oncol. 1993; 11:2351–2361. PMID: 8246024.

16. Hoskins PJ, Le N, Gascoyne RD, et al. Advanced diffuse large-cell lymphoma treated with 12-week combination chemotherapy: natural history of relapse after initial complete response and prognostic variables defining outcome after relapse. Ann Oncol. 1997; 8:1125–1132. PMID: 9426332.

17. Blay J, Gomez F, Sebban C, et al. The International Prognostic Index correlates to survival in patients with aggressive lymphoma in relapse: analysis of the PARMA trial. Parma Group. Blood. 1998; 92:3562–3568. PMID: 9808548.
18. Guglielmi C, Martelli M, Federico M, et al. Risk-assessment in diffuse large cell lymphoma at first relapse. A study by the Italian Intergroup for Lymphomas. Haematologica. 2001; 86:941–950. PMID: 11532622.
19. Jabbour E, Peslin N, Arnaud P, et al. Prognostic value of the age-adjusted International Prognostic Index in chemosensitive recurrent or refractory non-Hodgkin's lymphomas treated with high-dose BEAM therapy and autologous stem cell transplantation. Leuk Lymphoma. 2005; 46:861–867. PMID: 16019530.

20. Berglund M, Thunberg U, Amini RM, et al. Evaluation of immunophenotype in diffuse large B-cell lymphoma and its impact on prognosis. Mod Pathol. 2005; 18:1113–1120. PMID: 15920553.

21. Spaepen K, Stroobants S, Dupont P, et al. Prognostic value of positron emission tomography (PET) with fluorine-18 fluorodeoxyglucose ([18F]FDG) after first-line chemotherapy in non-Hodgkin's lymphoma: is [18F]FDG-PET a valid alternative to conventional diagnostic methods? J Clin Oncol. 2001; 19:414–419. PMID: 11208833.

22. Mikhaeel NG, Timothy AR, Hain SF, O'Doherty MJ. 18-FDG-PET for the assessment of residual masses on CT following treatment of lymphomas. Ann Oncol. 2000; 11(Suppl 1):147–150. PMID: 10707798.

Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download