1. Raab MS, Podar K, Breitkreutz I, Richardson PG, Anderson KC. Multiple myeloma. Lancet. 2009; 374:324–339. PMID:
19541364.

2. Park HJ, Park EH, Jung KW, et al. Statistics of hematologic malignancies in Korea: incidence, prevalence and survival rates from 1999 to 2008. Korean J Hematol. 2012; 47:28–38. PMID:
22479275.

3. Vallet S, Podar K. New insights, recent advances, and current challenges in the biological treatment of multiple myeloma. Expert Opin Biol Ther. 2013; 13(Suppl 1):S35–S53. PMID:
23768134.

4. Lee JH, Lee DS, Lee JJ, et al. Multiple Myeloma Working Party. Multiple myeloma in Korea: past, present, and future perspectives. Experience of the Korean Multiple Myeloma Working Party. Int J Hematol. 2010; 92:52–57. PMID:
20544403.

5. Kim K, Lee JH, Kim JS, et al. Clinical profiles of multiple myeloma in Asia-An Asian Myeloma Network study. Am J Hematol. 2014; 89:751–756. PMID:
24723430.

6. Kim SJ, Kim K, Kim BS, et al. Korean Multiple Myeloma Working Party. Clinical features and survival outcomes in patients with multiple myeloma: analysis of web-based data from the Korean Myeloma Registry. Acta Haematol. 2009; 122:200–210. PMID:
19887776.

7. Dimopoulos MA, Terpos E, Niesvizky R. How lenalidomide is changing the treatment of patients with multiple myeloma. Crit Rev Oncol Hematol. 2013; 88(Suppl 1):S23–S35. PMID:
23816163.

8. Mariz JM, Esteves GV. Review of therapy for relapsed/refractory multiple myeloma: focus on lenalidomide. Curr Opin Oncol. 2012; 24(Suppl 2):S3–S11. PMID:
22245806.
9. Larocca A, Cavallo F, Mina R, Boccadoro M, Palumbo A. Current treatment strategies with lenalidomide in multiple myeloma and future perspectives. Future Oncol. 2012; 8:1223–1238. PMID:
23130924.

11. Quach H, Ritchie D, Stewart AK, et al. Mechanism of action of immunomodulatory drugs (IMiDS) in multiple myeloma. Leukemia. 2010; 24:22–32. PMID:
19907437.

12. Ito T, Ando H, Suzuki T, et al. Identification of a primary target of thalidomide teratogenicity. Science. 2010; 327:1345–1350. PMID:
20223979.

13. Zhu YX, Kortuem KM, Stewart AK. Molecular mechanism of action of immune-modulatory drugs thalidomide, lenalidomide and pomalidomide in multiple myeloma. Leuk Lymphoma. 2013; 54:683–687. PMID:
22966948.

14. Chamberlain PP, Lopez-Girona A, Miller K, et al. Structure of the human Cereblon-DDB1-lenalidomide complex reveals basis for responsiveness to thalidomide analogs. Nat Struct Mol Biol. 2014; 21:803–809. PMID:
25108355.

15. Weber DM, Chen C, Niesvizky R, et al. Multiple Myeloma (009) Study Investigators. Lenalidomide plus dexamethasone for relapsed multiple myeloma in North America. N Engl J Med. 2007; 357:2133–2142. PMID:
18032763.

16. Dimopoulos M, Spencer A, Attal M, et al. Multiple Myeloma (010) Study Investigators. Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N Engl J Med. 2007; 357:2123–2132. PMID:
18032762.

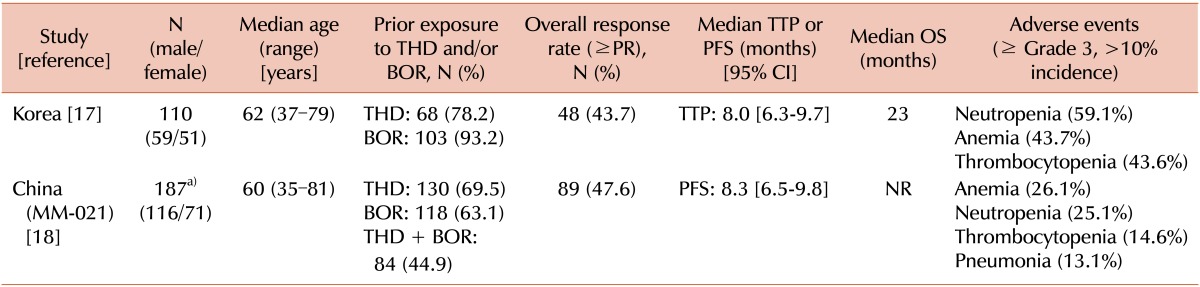
17. Kim K, Kim SJ, Voelter V, et al. Korean Multiple Myeloma Working Party (KMMWP). Lenalidomide with dexamethasone treatment for relapsed/refractory myeloma patients in Koreaexperience from 110 patients. Ann Hematol. 2014; 93:113–121. PMID:
24026427.

18. Hou J, Du X, Jin J, et al. A multicenter, open-label, phase 2 study of lenalidomide plus low-dose dexamethasone in Chinese patients with relapsed/refractory multiple myeloma: the MM-021 trial. J Hematol Oncol. 2013; 6:41. PMID:
23782711.

19. Iida S, Chou T, Okamoto S, et al. Lenalidomide plus dexamethasone treatment in Japanese patients with relapsed/refractory multiple myeloma. Int J Hematol. 2010; 92:118–126. PMID:
20559759.

20. Harousseau JL, Dreyling M. ESMO Guidelines Working Group. Multiple myeloma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010; 21(Suppl 5):v155–v157. PMID:
20555068.

21. Dimopoulos MA, Palumbo A, Attal M, et al. European Myeloma Network. Optimizing the use of lenalidomide in relapsed or refractory multiple myeloma: consensus statement. Leukemia. 2011; 25:749–760. PMID:
21293488.

22. Barosi G, Merlini G, Billio A, et al. SIE, SIES, GITMO evidence-based guidelines on novel agents (thalidomide, bortezomib, and lenalidomide) in the treatment of multiple myeloma. Ann Hematol. 2012; 91:875–888. PMID:
22476884.

23. Reece D, Kouroukis CT, Leblanc R, Sebag M, Song K, Ashkenas J. Practical approaches to the use of lenalidomide in multiple myeloma: a canadian consensus. Adv Hematol. 2012; 2012:621958. PMID:
23097669.

24. Chen C, Baldassarre F, Kanjeekal S, Herst J, Hicks L, Cheung M. Lenalidomide in multiple myeloma-a practice guideline. Curr Oncol. 2013; 20:e136–e149. PMID:
23559881.

25. Lonial S. Relapsed multiple myeloma. Hematology Am Soc Hematol Educ Program. 2010; 2010:303–309. PMID:
21239810.

26. Dimopoulos MA, Chen C, Spencer A, et al. Long-term follow-up on overall survival from the MM-009 and MM-010 phase III trials of lenalidomide plus dexamethasone in patients with relapsed or refractory multiple myeloma. Leukemia. 2009; 23:2147–2152. PMID:
19626046.

27. Palumbo A, Mateos MV, Bringhen S, San Miguel JF. Practical management of adverse events in multiple myeloma: can therapy be attenuated in older patients? Blood Rev. 2011; 25:181–191. PMID:
21497966.

28. Stadtmauer EA, Weber DM, Niesvizky R, et al. Lenalidomide in combination with dexamethasone at first relapse in comparison with its use as later salvage therapy in relapsed or refractory multiple myeloma. Eur J Haematol. 2009; 82:426–432. PMID:
19302559.

29. Wang M, Dimopoulos MA, Chen C, et al. Lenalidomide plus dexamethasone is more effective than dexamethasone alone in patients with relapsed or refractory multiple myeloma regardless of prior thalidomide exposure. Blood. 2008; 112:4445–4451. PMID:
18799726.

30. Katodritou E, Vadikolia C, Lalagianni C, et al. "Real-world" data on the efficacy and safety of lenalidomide and dexamethasone in patients with relapsed/refractory multiple myeloma who were treated according to the standard clinical practice: a study of the Greek Myeloma Study Group. Ann Hematol. 2014; 93:129–139. PMID:
23892921.

31. San-Miguel JF, Dimopoulos MA, Stadtmauer EA, et al. Effects of lenalidomide and dexamethasone treatment duration on survival in patients with relapsed or refractory multiple myeloma treated with lenalidomide and dexamethasone. Clin Lymphoma Myeloma Leuk. 2011; 11:38–43. PMID:
21273172.

32. Harousseau JL, Dimopoulos MA, Wang M, et al. Better quality of response to lenalidomide plus dexamethasone is associated with improved clinical outcomes in patients with relapsed or refractory multiple myeloma. Haematologica. 2010; 95:1738–1744. PMID:
20460639.

33. Zago M, Oehrlein K, Rendl C, Hahn-Ast C, Kanz L, Weisel K. Lenalidomide in relapsed and refractory multiple myeloma disease: feasibility and benefits of long-term treatment. Ann Hematol. 2014; 93:1993–1999. PMID:
24974802.

34. Fouquet G, Tardy S, Demarquette H, et al. Efficacy and safety profile of long-term exposure to lenalidomide in patients with recurrent multiple myeloma. Cancer. 2013; 119:3680–3686. PMID:
23921945.

35. Cerrato C, Mina R, Palumbo A. Optimal management of elderly patients with myeloma. Expert Rev Anticancer Ther. 2014; 14:217–228. PMID:
24308685.

37. Chanan-Khan AA, Lonial S, Weber D, et al. Lenalidomide in combination with dexamethasone improves survival and time-to-progression in patients ≥65 years old with relapsed or refractory multiple myeloma. Int J Hematol. 2012; 96:254–262. PMID:
22752567.
38. Rajkumar SV, Jacobus S, Callander NS, et al. Eastern Cooperative Oncology Group. Lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone as initial therapy for newly diagnosed multiple myeloma: an open-label randomised controlled trial. Lancet Oncol. 2010; 11:29–37. PMID:
19853510.

39. Chen N, Wen L, Lau H, Surapaneni S, Kumar G. Pharmacokinetics, metabolism and excretion of [(14)C]-lenalidomide following oral administration in healthy male subjects. Cancer Chemother Pharmacol. 2012; 69:789–797. PMID:
22037879.

40. Dimopoulos M, Alegre A, Stadtmauer EA, et al. The efficacy and safety of lenalidomide plus dexamethasone in relapsed and/or refractory multiple myeloma patients with impaired renal function. Cancer. 2010; 116:3807–3814. PMID:
20564094.

41. Dimopoulos MA, Terpos E, Goldschmidt H, Alegre A, Mark T, Niesvizky R. Treatment with lenalidomide and dexamethasone in patients with multiple myeloma and renal impairment. Cancer Treat Rev. 2012; 38:1012–1019. PMID:
22609463.

42. Dimopoulos MA, Christoulas D, Roussou M, et al. Lenalidomide and dexamethasone for the treatment of refractory/relapsed multiple myeloma: dosing of lenalidomide according to renal function and effect on renal impairment. Eur J Haematol. 2010; 85:1–5. PMID:
20192988.

43. Oehrlein K, Langer C, Sturm I, et al. Successful treatment of patients with multiple myeloma and impaired renal function with lenalidomide: results of 4 German centers. Clin Lymphoma Myeloma Leuk. 2012; 12:191–196. PMID:
22341857.

44. Tosi P, Gamberi B, Castagnari B, et al. Lenalidomide in combination with dexamethasone in elderly patients with advanced, relapsed or refractory multiple myeloma and renal failure. Mediterr J Hematol Infect Dis. 2013; 5:e2013037. PMID:
23795275.
45. Palumbo A, Bladé J, Boccadoro M, et al. How to manage neutropenia in multiple myeloma. Clin Lymphoma Myeloma Leuk. 2012; 12:5–11. PMID:
22178143.

46. Bennett CL, Angelotta C, Yarnold PR, et al. Thalidomide- and lenalidomide-associated thromboembolism among patients with cancer. JAMA. 2006; 296:2558–2560. PMID:
17148721.

47. Carrier M, Le Gal G, Tay J, Wu C, Lee AY. Rates of venous thromboembolism in multiple myeloma patients undergoing immunomodulatory therapy with thalidomide or lenalidomide: a systematic review and meta-analysis. J Thromb Haemost. 2011; 9:653–663. PMID:
21255254.

48. Ishak J, Dimopoulos MA, Weber D, Knight RD, Shearer A, Caro JJ. Declining rates of adverse events and dose modifications with lenalidomide in combination with dexamethasone. Blood. 2008; 112:abst 3708. ASH Annual Meeting.

49. Jang MJ, Bang SM, Oh D. Incidence of venous thromboembolism in Korea: from the Health Insurance Review and Assessment Service database. J Thromb Haemost. 2011; 9:85–91. PMID:
20942850.

50. Lee KW, Bang SM, Kim S, et al. The incidence, risk factors and prognostic implications of venous thromboembolism in patients with gastric cancer. J Thromb Haemost. 2010; 8:540–547. PMID:
20040044.

51. Kang MJ, Ryoo BY, Ryu MH, et al. Venous thromboembolism (VTE) in patients with advanced gastric cancer: an Asian experience. Eur J Cancer. 2012; 48:492–500. PMID:
22169121.

52. Choi S, Lee KW, Bang SM, et al. Different characteristics and prognostic impact of deep-vein thrombosis / pulmonary embolism and intraabdominal venous thrombosis in colorectal cancer patients. Thromb Haemost. 2011; 106:1084–1094. PMID:
22072215.

53. Sun JM, Kim TS, Lee J, et al. Unsuspected pulmonary emboli in lung cancer patients: the impact on survival and the significance of anticoagulation therapy. Lung Cancer. 2010; 69:330–336. PMID:
20007002.

54. Koh Y, Bang SM, Lee JH, et al. Korean Multiple Myeloma Working Party. Low incidence of clinically apparent thromboembolism in Korean patients with multiple myeloma treated with thalidomide. Ann Hematol. 2010; 89:201–206. PMID:
19705118.

55. Wu SY, Yeh YM, Chen YP, Su WC, Chen TY. Low incidence of thromboembolism in relapsed/refractory myeloma patients treated with thalidomide without thromboprophylaxis in Taiwan. Ann Hematol. 2012; 91:1773–1778. PMID:
22706703.

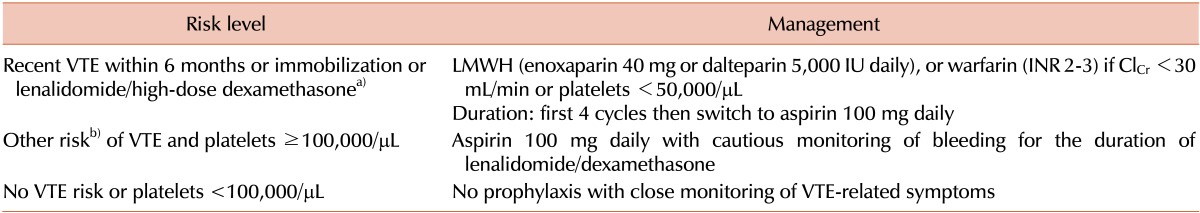
56. Palumbo A, Rajkumar SV, Dimopoulos MA, et al. International Myeloma Working Group. Prevention of thalidomide- and lenalidomide-associated thrombosis in myeloma. Leukemia. 2008; 22:414–423. PMID:
18094721.

57. Chen C, Reece DE, Siegel D, et al. Expanded safety experience with lenalidomide plus dexamethasone in relapsed or refractory multiple myeloma. Br J Haematol. 2009; 146:164–170. PMID:
19545290.

58. Amin RP, Fuchs A, Christian MS, et al. An embryo-fetal developmental toxicity study of lenalidomide in cynomolgus monkeys. Birth Defects Res A Clin Mol Teratol. 2009; 85(Suppl):435.
59. Chen N, Lau H, Choudhury S, Wang X, Assaf M, Laskin OL. Distribution of lenalidomide into semen of healthy men after multiple oral doses. J Clin Pharmacol. 2010; 50:767–774. PMID:
20160158.

60. Lonial S, Baz R, Swern AS, Weber D, Dimopoulos MA. Neutropenia is a predictable and early event in affected patients with relapsed/refractory multiple myeloma treated with lenalidomide in combination with dexamethasone. Blood. 2009; 114 (Ash Annual Meeting):abst 2879.

61. Zangari M, Tricot G, Polavaram L, et al. Survival effect of venous thromboembolism in patients with multiple myeloma treated with lenalidomide and high-dose dexamethasone. J Clin Oncol. 2010; 28:132–135. PMID:
19901114.

62. Lee AY. Anticoagulation in the treatment of established venous thromboembolism in patients with cancer. J Clin Oncol. 2009; 27:4895–4901. PMID:
19738121.

63. Kearon C, Akl EA, Comerota AJ, et al. American College of Chest Physicians. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012; 141(2 Suppl):e419S–e494S. PMID:
22315268.
65. Dimopoulos MA, Orlowski RZ, Niesvizky R, Lonial S, Brandenburg NA, Weber DM. Lenalidomide and dexamethasone (LEN plus DEX) treatment in relapsed/refractory multiple myeloma (RRMM) patients (pts) and risk of second primary malignancies (SPM): Analysis of MM-009/010. J Clin Oncol. 2011; 29(15 Suppl):abst 8009.

66. Morgan G, Durie B, San Miguel J, et al. Retrospective analysis of the long term safety of lenalidomide (LEN) ± dexamethasone (DEX) in relapsed/refractory multiple myeloma (RRMM) patients (PTS): analysis of pooled data and incidence rates (IR) of second primary malignancy (SPM). Haematologica. 2011; 96(Suppl 1):S24.
67. Hussain S, Browne R, Chen J, Parekh S. Lenalidomide-induced severe hepatotoxicity. Blood. 2007; 110:3814. PMID:
17984315.

68. Figaro MK, Clayton W Jr, Usoh C, et al. Thyroid abnormalities in patients treated with lenalidomide for hematological malignancies: results of a retrospective case review. Am J Hematol. 2011; 86:467–470. PMID:
21544854.

69. João C, Coelho I, Costa C, Esteves S, Lucio P. Efficacy and safety of lenalidomide in relapse/refractory multiple myeloma--real life experience of a tertiary cancer center. Ann Hematol. 2015; 94:97–105. PMID:
25038919.

70. Yang B, Yu RL, Chi XH, Lu XC. Lenalidomide treatment for multiple myeloma: systematic review and meta-analysis of randomized controlled trials. PLoS One. 2013; 8:e64354. PMID:
23691202.

71. Huang SY, Yao M, Tang JL, et al. Epidemiology of multiple myeloma in Taiwan: increasing incidence for the past 25 years and higher prevalence of extramedullary myeloma in patients younger than 55 years. Cancer. 2007; 110:896–905. PMID:
17594697.
72. Tan D, Chng WJ, Chou T, et al. Management of multiple myeloma in Asia: resource-stratified guidelines. Lancet Oncol. 2013; 14:e571–e581. PMID:
24176575.

73. Richardson P, Mitsiades C, Laubach J, et al. Lenalidomide in multiple myeloma: an evidence-based review of its role in therapy. Core Evid. 2010; 4:215–245. PMID:
20694078.

74. Popat R, Khan I, Dickson J, et al. An alternative dosing strategy of lenalidomide for patients with relapsed multiple myeloma. Br J Haematol. 2015; 168:148–151. PMID:
25103844.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download