TO THE EDITOR: Granulocyte colony-stimulating factor (G-CSF)-associated blastocytosis with a lack of an associated left shift poses a challenge for accurate assessment of the underlying disease [1]. In particular, blastocytosis after chemotherapy for acute leukemia may mimic a recurrence of underlying leukemia. Only a few cases of myeloblastosis in AML or ALL or of leukoerythroblastosis in AML after G-CSF therapy have been described anecdotally in the literature [2,3,4]. Herein, we report a case of myeloblastosis in ALL within 2 days of G-CSF administration masquerading as early relapse, causing a diagnostic dilemma. This was compounded by a striking lack of telltale evidence that G-CSF treatment was effective, such as increased granulation and a left shift.
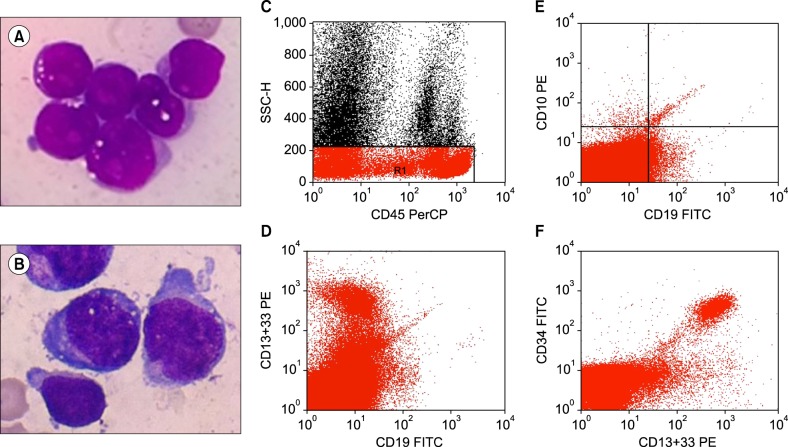
A 25-year-old man diagnosed with common acute lymphoblastic leukemia antigen [CALLA] positive, precursor B-cell ALL (Fig. 1A) completed his first phase of induction chemotherapy with methotrexate, cyclophosphamide, and cytarabine. His day 36 bone marrow showed 2.8% blasts with positive minimal residual disease (MRD) (0.046%). To accelerate recovery from persistent neutropenia, he was treated with G-CSF; an adequate response was noted, with a total leukocyte count (TLC) of 2.9×109/L. On day 70, he completed his second phase of induction chemotherapy and was started on G-GSF therapy for severe neutropenia (TLC: 0.76×109/L). On day 73, his peripheral blood smear showed marked leukopenia with a predominance of lymphocytes and 2% blasts without any left shift or increased granulations. This lack of prototypical G-CSF response along with previous MRD positivity and presence of 2% blasts led to a suspicion of relapse, and bone marrow examination was advised. Marrow aspirate showed hypocellular marrow with 14% blasts, and focal areas showed up to 25% blasts (Fig. 1B), along with a lack of a maturing myeloid component, which suggested relapse. Trephine biopsy, in contrast, showed hypocellularity with only a few scattered CD34 positive blasts. This necessitated flow cytometric evaluation, where the blasts showed negativity for B lymphoblastic markers and expressed myeloid markers, including CD13, CD33, and CD34, suggesting that they were regenerating myeloblasts (Fig. 1C-F). No further therapy was administered and the patient was kept on close hematological follow-up. The counts recovered after 4 days, with the typical responses of a left shift and increased granulation. Follow-up marrow examination after complete recovery of blood counts, 10 days after discontinuing G-CSF therapy, revealed cellular marrow elements with 4% blasts (regenerating) and negative MRD (<0.01%).
Post-G-CSF blastocytosis is a problematic and confusing issue that is difficult to differentiate from disease recurrence and hemopoietic recovery. In our case, there were regenerating blasts (>20%) in the bone marrow, with an absence of telltale G-CSF-induced changes, such as presence of immature granulocytes, monocytosis, or prominent granulations on peripheral smear. To conclude, the regenerating blast percentage may increase after G-CSF therapy to >20%, without concomitant increases in other mature myeloid precursors.
The lag time before complete response to G-CSF therapy may be as long as 10 days, as seen in our case, posing potential problems in management and a challenge in therapeutic decisions. A careful morphological assessment of the blasts at the time of diagnosis, aided by awareness of these G-CSF-associated aberrant patterns and flow cytometric characterization can help guide management decisions.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download