TO THE EDITOR: The concept of mucosa-associated lymphoid tissue (MALT) lymphoma was first proposed by Isaacson and Wright in 1983 and is now recognized as a distinct clinical-pathologic disease entity [1]. Between 60% and 70% of patients with MALT lymphomas present with localized (stage I or II) disease involving a non-lymphatic organ. MALT lymphoma arising from the salivary glands is rare; data in the literature are scarce, limited to small series and isolated case reports. Therefore, the characteristics and clinical outcome of this unusual presentation are largely unknown. Extranodal marginal zone lymphoma has a slight female preponderance and usually presents at a localized stage with a low incidence of widespread dissemination, thus requiring local treatment, such as radiation therapy (RT), rather than systemic therapy [2]. In the present case report, we describe a case of MALT lymphoma involving both salivary glands presenting with Sjögren's syndrome and renal tubular acidosis.
A 42-year-old woman diagnosed with renal tubular acidosis type II, secondary to Fanconi's syndrome, presented with swelling in the left cheek that was not tender but was progressively increasing in size (Fig. 1). Physical examination revealed palpable bilateral swelling in the region of the angle of the mandible measuring 5×5 cm on the left side and 3×3 cm on the right side. The swelling was firm and fixed to the underlying structures. There were no palpable cervical lymph nodes or any other swelling. Ultrasonography revealed a bulky heterogeneous lesion in the left parotid gland measuring approximately 10 mL, suggesting parotitis or a parotid gland abscess. She was given a course of antibiotics for a week but the swelling did not resolve after the treatment. Fine needle aspiration cytology (FNAC) of the swelling showed numerous small-sized lymphoid cells in varying stages of maturation along with histiocytes, indicating a parotid lymph node.
Contrast enhanced magnetic resonance imaging (MRI) of the face and neck revealed complete replacement of the entire left parotid parenchyma by a large, solid lesion involving both the superficial and deep lobes measuring 5.8×4.2×4.0 cm (Fig. 2). A similar lesion was visualized in the right parotid gland measuring 3×2×2 cm with enlarged cervical lymph nodes, which suggested the diagnosis of lymphoma or Warthin's tumor.
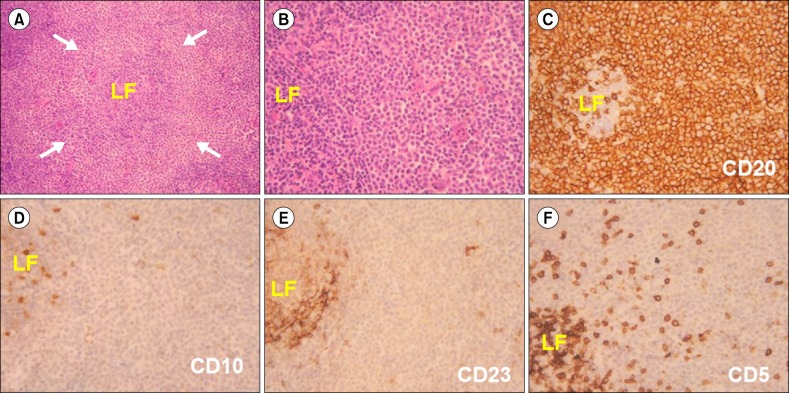
A trucut biopsy of the left parotid gland swelling was performed, which revealed a moderate amount of sheets of monotonous lymphoid cells with round irregular nuclei, coarsely clumped chromatin and inconspicuous nuclei with eosinophilic cytoplasm with occasional mitotic figures, resembling monocytoid B cells. Immunohistochemically, the cells were positive for CD20 and Bcl-2, weakly positive for Bcl-6 with a Ki-67 proliferative index of 35%, suggestive of MALT lymphoma (Fig. 3).
There was no evidence of lymphadenopathy, extra-nodal spread or bone marrow involvement (stage IE). The serology test for hepatitis B virus & hepatitis C virus (HCV), were negative, and did not have past history suggestive of any autoimmune disorder.
In consideration of the limited stage disease, she was scheduled for localized radiation therapy with three-dimensional conformal radiation therapy. She received a total dose of 36 Gy in 12 fractions over 16 days to the right and left parotid area by utilizing paired tangential fields. She achieved a complete clinical and radiological response. She is being followed-up monthly and has been disease-free for 32 months.
Primary lymphomas of the salivary glands are rare and account for 4.7% of all lymphomas [3]. A non-Hodgkin lymphoma of the salivary gland may appear as a painless, but progressively enlarging mass [4]. Therefore, it is rarely suspected, leading to a delay in diagnosis.
The native absence of MALT within the salivary glands necessitates the development of acquired MALT from underlying lymphoid stimulation and infiltration before MALT lymphoma can develop [5]. Low-grade MALTomas of the parotid gland usually arise in the setting of a benign lymphoepithelial lesion (BLL) [6]. The transformation from BLL to MALToma is believed to be a multi-step process. The initial event in this process may be a long-term stimulation of activating B cells by an inflammatory stimulus [7].
Rosenstiel et al. [8] found that patients with Sjögren's syndrome have a 44-fold increased risk of developing non-Hodgkin lymphoma, and 80% of these lymphomas are of the MALT-type. Our patient had also presented with clinical evidence of Sjögren's syndrome. The MALT lymphoma often affects middle-aged adults, with a median age of 50 years, and the parotid gland is by far the most commonly involved site, sometimes bilaterally. Patients typically present with a painless mass. Anacak et al. [9] reported salivary gland MALToma to be a disease primarily affecting women; in their study, the ratio of women to men was 3:1. An association between HCV infection and B-cell lymphomas has previously been reported, especially in countries in which the prevalence of HCV is relatively high [10].
FNAC of this type of lesion is usually inconclusive, often delaying diagnosis. Biopsy and IHC can confirm the diagnosis with absolute certainty [11]. Contrast enhanced computed tomography (CT) imaging studies of the chest, abdomen and pelvis are essential to stage the disease accurately [12]. Currently, there is a controversy in the reported literature regarding the accuracy of positron-emission tomography-CT scanning of MALTomas.
Radical parotidectomy is not indicated because of the associated morbidity, and because RT alone can secure local control and allow tissue preservation. Low dose RT (30 Gy) is extremely efficacious for local control of the disease, with local control rates ranging from 97% to 100%; 5-year progression free survival and overall survival are approximately 76% and 91%, respectively [13].
Regional and distant relapses are not common in gastric MALT lymphomas, but extragastric MALTomas tend to be more aggressive and may recur in the regional or distant lymph nodes and in other organs [1415]. According to Wenzel et al., patients with MALToma of the head and neck are at a relatively high risk for early dissemination and subsequent distant recurrence when only local therapies are applied. In the current case, there was no lymph node or other organ involvement.
Due to the high local control rate and low morbidity, together with the indolent biology of the disease, we conclude that moderate-dose RT (25 to 30 Gy) is a safe and effectivetreatment option for stage I and II MALTomas in the parotid gland.
References
1. Harris NL, Jaffe ES, Stein H, et al. A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood. 1994; 84:1361–1392. PMID: 8068936.
2. Thieblemont C, Berger F, Dumontet C, et al. Mucosa-associated lymphoid tissue lymphoma is a disseminated disease in one third of 158 patients analyzed. Blood. 2000; 95:802–806. PMID: 10648389.

3. Balm AJ, Delaere P, Hilgers FJ, Somers R, Van Heerde P. Primary lymphoma of mucosa associated lymphoid tissue (MALT) in the parotid gland. Clin Otolaryngol Allied Sci. 1993; 18:528–532. PMID: 8877235.

4. Mehle ME, Kraus DH, Wood BG, Tubbs R, Tucker HM, Lavertu P. Lymphoma of the parotid gland. Laryngoscope. 1993; 103:17–21. PMID: 8421414.

5. Ciccone E, Truini M, Grossi CE. Lymphoid complement of the human salivary glands: function and pathology. Eur J Morphol. 1998; 36(Suppl):252–256. PMID: 9825932.
6. Diss TC, Wotherspoon AC, Speight P, Pan L, Isaacson PG. B-cell monoclonality, Epstein Barr virus, and t(14;18) in myoepithelial sialadenitis and low-grade B-cell MALT lymphoma of the parotid gland. Am J Surg Pathol. 1995; 19:531–536. PMID: 7726362.
7. Marioni G, Marchese-Ragona R, Marino F, et al. MALT-type lymphoma and Warthin's tumour presenting in the same parotid gland. Acta Otolaryngol. 2004; 124:318–323. PMID: 15141762.

8. Rosenstiel DB, Carroll WR, Listinsky CM. MALT lymphoma presenting as a cystic salivary gland mass. Head Neck. 2001; 23:254–258. PMID: 11428457.

9. Anacak Y, Miller RC, Constantinou N, et al. Primary mucosa-associated lymphoid tissue lymphoma of the salivary glands: a multicenter Rare Cancer Network study. Int J Radiat Oncol Biol Phys. 2012; 82:315–320. PMID: 21075560.

10. Ferri C, Caracciolo F, Zignego AL, et al. Hepatitis C virus infection in patients with non-Hodgkin's lymphoma. Br J Haematol. 1994; 88:392–394. PMID: 7803287.

11. Ando M, Matsuzaki M, Murofushi T. Mucosa-associated lymphoid tissue lymphoma presented as diffuse swelling of the parotid gland. Am J Otolaryngol. 2005; 26:285–288. PMID: 15991099.

12. Perry C, Herishanu Y, Metzer U, et al. Diagnostic accuracy of PET/CT in patients with extranodal marginal zone MALT lymphoma. Eur J Haematol. 2007; 79:205–209. PMID: 17662066.

13. Tsai HK, Li S, Ng AK, Silver B, Stevenson MA, Mauch PM. Role of radiation therapy in the treatment of stage I/II mucosa-associated lymphoid tissue lymphoma. Ann Oncol. 2007; 18:672–678. PMID: 17218489.

14. Wenzel C, Fiebiger W, Dieckmann K, Formanek M, Chott A, Raderer M. Extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue of the head and neck area: high rate of disease recurrence following local therapy. Cancer. 2003; 97:2236–2241. PMID: 12712477.
15. Zinzani PL, Magagnoli M, Ascani S, et al. Nongastrointestinal mucosa-associated lymphoid tissue (MALT) lymphomas: clinical and therapeutic features of 24 localized patients. Ann Oncol. 1997; 8:883–886. PMID: 9358939.

Fig. 1
(A) Left parotid swelling measuring 5.8×4.2×4 cm at initial diagnosis. (B) Complete clinical response after radiotherapy.

Fig. 2
Contrast enhanced T1- weighted axial and coronal magnetic resonance imaging showing a 5.8×4.2×4 cm lesion replacing the left parotid gland and a 3×2×2 cm lesion in the right parotid gland (A, C). T2-weighted axial and coronal images showing homogenously hyperintense lesions in both parotid glands (B, D).

Fig. 3
(A) Expanded pale looking marginal zone (arrows) surrounding a lymphoid follicle (LF), H&E ×100. (B) Marginal zone cells with round to slightly irregular nuclei and moderate amount of pale cytoplasm, H&E ×400. (C) to (F) Immunohistochemistry showing a CD20+, CD10-, CD23- and CD5-neoplastic lymphoid cells. DAB chromogen ×400.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download