TO THE EDITOR: [Abstract] The treatment outcome of relapsed or refractory extranodal natural killer (NK)/T-cell lymphoma (ENKL) is poor. Brentuximab vedotin, an anti-CD30 antibody-drug conjugate, has recently been approved for the treatment of relapsed Hodgkin's lymphoma and anaplastic large-cell lymphoma (ALCL). We report on a case of a 63-year-old man who presented with multiple skin lesions, and was diagnosed with ENKL. Since the disease was refractory to most chemotherapy drugs, we performed an analysis of the skin biopsy to evaluate marker CD30. The patient's lymphoma cells demonstrated CD30-positivity, and treatment with single-agent brentuximab vedotin was commenced as of December 2013. Following 4 cycles of single-agent brentuximab vedotin treatment, all of the skin lesions had cleared, and a [18F]-fluorodeoxyglucose (FDG)-positron emission tomography (PET)/computed tomography (CT) scan demonstrated complete remission (CR) of the disease. This study suggests that single-agent brentuximab vedotin could be effective in the treatment of CD30-positive non-Hodgkin lymphomas other than ALCL.
Extranodal natural killer (NK)/T-cell lymphoma (ENKL) is an aggressive, non-Hodgkin lymphoma for which a standard therapy has not yet been established. The treatment outcomes of advanced relapsed or refractory ENKL, with conventional chemotherapy, are extremely poor [1]. Brentuximab vedotin (Seattle Genetics Inc., Bothell, WA, USA) is an anti-CD30 antibody-drug conjugate that is covalently linked, via a protease-cleavable linker, to the microtubule-disrupting agent, monomethyl auristatin E.
In a phase 1 clinical trial of 45 patients with relapsed or refractory CD30-positive lymphomas, treatment with single-agent brentuximab vedotin resulted in an overall response rate (ORR) of 67% [2]. In a phase 2 clinical trial for relapsed or refractory systemic anaplastic large-cell lymphoma (ALCL), single-agent brentuximab vedotin treatment resulted in an ORR of 86%, and a complete remission (CR) rate of 57% [3]. Based on these results, brentuximab vedotin was approved by the United States Food and Drug Administration, in August 2011, for the treatment of relapsed Hodgkin's lymphoma and ALCL. In a recent phase 2 clinical trial for relapsed T-cell lymphoma, treatment with single-agent brentuximab vedotin resulted in an ORR of 41% in relapsed T-cell lymphoma and 54% in angioimmunoblastic T-cell lymphoma patients, respectively [4]. Therefore, these studies demonstrate that single-agent brentuximab vedotin treatment in non-Hodgkin lymphoma patients with tumors expressing CD30 can induce objective responses. Here, we report on a case of a 63-year-old man with refractory CD30-positive ENKL in which CR was achieved with single-agent brentuximab vedotin treatment.
A 63-year-old man presented with back pain in April 2011. A computed tomography (CT) scan revealed enlargement of his retroperitoneal lymph nodes. The patient underwent excisional biopsy and was diagnosed with malignant lymphoma. He was heavily pretreated: with cyclophosphamide, doxorubicin, vincristine, and prednisolone (3 cycles); ifosfamide, methotrexate, etoposide, and prednisolone plus L-asparaginase (1 cycle); rituximab with dose-modified cyclophosphamide, vincristine, doxorubicin, methotrexate/etoposide, ifosfamide, and cytarabine (1 cycle); and gemcitabine with dexamethasone (1 cycle). During treatment, new skin lesions developed at multiple sites, including the face, trunk, arms, and legs, with a skin biopsy revealing malignant lymphoma. The patient was subsequently treated with 5 cycles of bortezomib in combination with dexamethasone, and 4 cycles of pralatrexate (20 mg once a week, every 4 weeks). However, the disease still progressed.
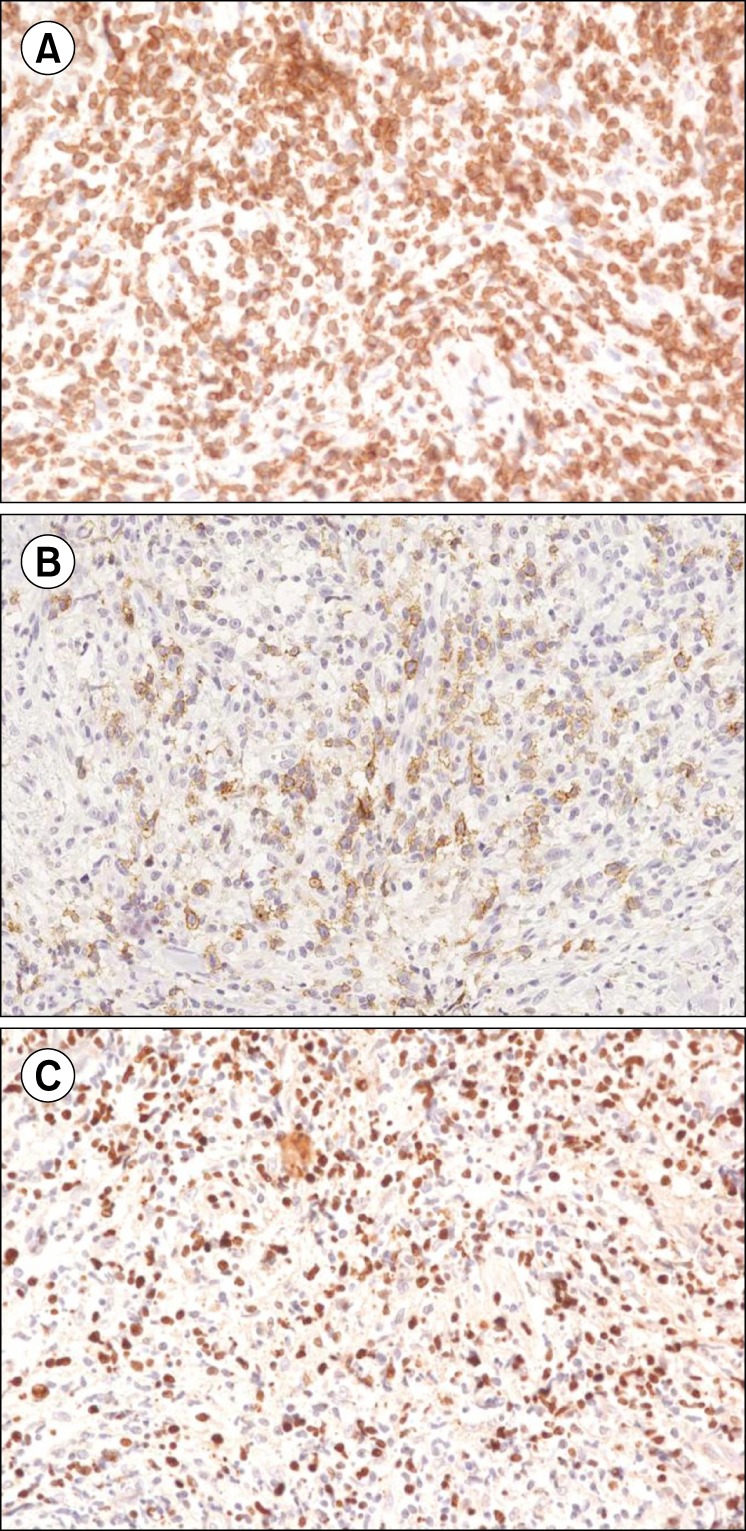
Despite the extensive treatment, the disease proved refractory to most chemotherapeutic agents. The skin lesions initially manifested as multiple papules and nodules, which gradually developed into ulcerative lesions (Fig. 1A, B). Since brentuximab vedotin was available at the time, we analyzed the skin biopsy performed in January 2012 to evaluate CD30-positivity. The skin biopsy demonstrated the tumor to be an ENKL positive for CD3 (Fig. 2A), CD30 (Fig. 2B), and Epstein-Barr virus in situ hybridization (Fig. 2C). Informed, written consent was obtained prior to treatment with single-agent brentuximab vedotin. In December 2013, treatment commenced and the patient received a 1.8 mg/kg dose of single-agent brentuximab vedotin, administered intravenously every 3 weeks. From December 2013 to March 2014, the patient received 4 cycles of single-agent brentuximab vedotin treatment. After 4 cycles, all of the skin lesions had cleared, and a [18F]-fluorodeoxyglucose (FDG)-positron emission tomography (PET)/CT scan showed CR of the disease (Fig. 1C, D). The patient developed grade 2 toxicity dyspnea with no other significant adverse events. Treatment was stopped for 3 months due to the dyspnea and relapse was observed in May 2014.
ENKL is usually derived from NK cells or, in rare instances, from cytotoxic T-cells. NK/T-cell lymphomas are typically positive for CD2, CD56, and cytoplasmic CD3 [5]. CD30-positivity is known to be associated with Hodgkin's lymphoma, but it is also detected in ALCL, some T- and B-cell lymphomas, and embryonal cell carcinomas [6]. In a study of patients with ENKL, 36.4% were CD30-positive, exhibiting reduced event free survival and overall survival compared to those without CD30 expression [7]. For patients with refractory ENKL, benefits may be anticipated with L-asparaginase-based combination chemotherapy, but it is not an established therapy [8].
A recent phase 2 clinical trial by Horwitz et al proved that single-agent brentuximab vedotin could be a viable therapeutic option for relapsed and refractory peripheral T-cell lymphoma [4]. However, there has been no study or clinical trial, to date, evaluating the use of single-agent brentuximab vedotin in ENKL. We report a case where single-agent brentuximab vedotin induced CR that was long lasting in a patient with refractory CD30-positive ENKL. The only toxicity of brentuximab vedotin treatment in this patient was dyspnea of grade 2, with other studies having also reported manageable toxicities. Among the most common adverse events were dose-related sensory peripheral neuropathy, nausea, neutropenia, diarrhea, and pyrexia, with lung toxicity occurring very rarely [9].
In conclusion, we suggest that single-agent brentuximab vedotin could be a viable treatment option for refractory CD30-positive ENKL without causing significant toxicity, even in heavily pretreated patients. However, since this case report is the first to show CR in CD30-positive ENKL with brentuximab vedotin, further studies will be required to confirm these results and establish a dosing schedule. This report implies that brentuximab vedotin could be effective in treating CD30-positive non-Hodgkin lymphoma other than ALCL and peripheral T-cell lymphoma.
References
1. Yamaguchi M, Suzuki R, Kwong YL, et al. Phase I study of dexamethasone, methotrexate, ifosfamide, L-asparaginase, and etoposide (SMILE) chemotherapy for advanced-stage, relapsed or refractory extranodal natural killer (NK)/T-cell lymphoma and leukemia. Cancer Sci. 2008; 99:1016–1020. PMID: 18294294.

2. Younes A, Bartlett NL, Leonard JP, et al. Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. N Engl J Med. 2010; 363:1812–1821. PMID: 21047225.

3. Pro B, Advani R, Brice P, et al. Brentuximab vedotin (SGN-35) in patients with relapsed or refractory systemic anaplastic large-cell lymphoma: results of a phase II study. J Clin Oncol. 2012; 30:2190–2196. PMID: 22614995.

4. Horwitz SM, Advani RH, Bartlett NL, et al. Objective responses in relapsed T-cell lymphomas with single-agent brentuximab vedotin. Blood. 2014; 123:3095–3100. PMID: 24652992.

5. Kwong YL. Natural killer-cell malignancies: diagnosis and treatment. Leukemia. 2005; 19:2186–2194. PMID: 16179910.

6. Falini B, Pileri S, Pizzolo G, et al. CD30 (Ki-1) molecule: a new cytokine receptor of the tumor necrosis factor receptor superfamily as a tool for diagnosis and immunotherapy. Blood. 1995; 85:1–14. PMID: 7803786.

7. Hong J, Park S, Baek HL, et al. Tumor cell nuclear diameter and CD30 expression as potential prognostic parameter in patients with extranodal NK/T-cell lymphoma, nasal type. Int J Clin Exp Pathol. 2012; 5:939–947. PMID: 23119111.
9. Katz J, Janik JE, Younes A. Brentuximab vedotin (SGN-35). Clin Cancer Res. 2011; 17:6428–6436. PMID: 22003070.

Fig. 1
(A, B) Multiple ulcerative skin lesions before treatment with brentuximab vedotin. Positron emission tomography (PET) scan (C) before, and (D) after 4 cycles of treatment with single-agent brentuximab vedotin.
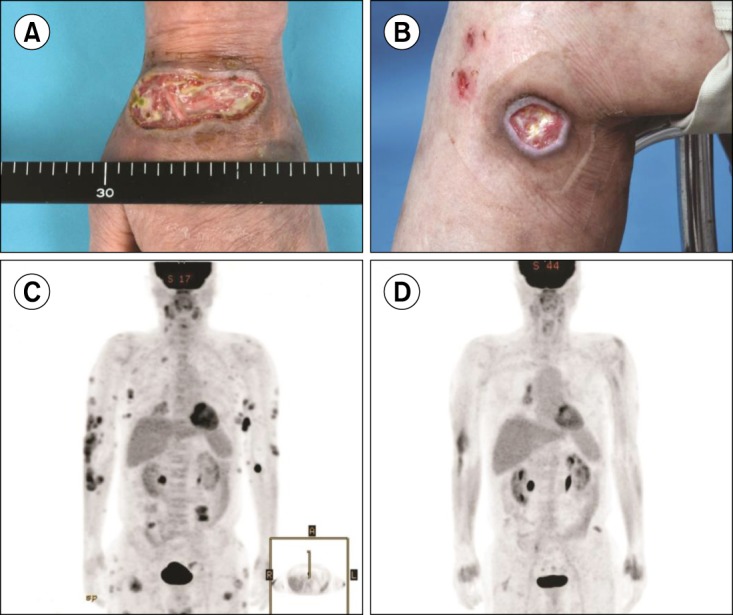
Fig. 2
Pathology images of the skin biopsy showing extranodal NK/T-cell lymphoma (ENKL). (A) Positive staining for CD3 in lymphoma cells (×400 magnification). (B) Positive staining for CD30 in lymphoma cells (×400 magnification). (C) In situ hybridization of Epstein-Barr virus, showing positive cells (×400 magnification).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download