Abstract
Background
Large-scale epidemiologic analysis for hematologic malignancies will be helpful to understand the trends in incidence and survival.
Methods
The Korea Central Cancer Registry (KCCR) updated the nationwide analysis on the incidence and survival of myeloid malignancies, from the Korean National Cancer Incidence Database between 1999 and 2012. Myeloid malignancies were classified based on the International Classification of Diseases for Oncology 3rd edition (ICD-O-3).
Results
Overall 3,771 cases of myeloid diseases, which was 1.7% of all cancers, were identified in 2012. The highest incidence of myeloid malignancies was observed in age 70s and male predominance was noted (1.3:1). Acute myeloid leukemia (AML) was the most frequent subtype, followed by myeloproliferative neoplasms (MPN), myelodysplastic syndrome (MDS) and MDS/MPN: age-standardized incidence rates (ASR) in 2012 for each disease were 2.02, 1.95, 1.13, and 0.12 per 100,000 persons, respectively. The ASR for all myeloid malignancies was increased from 3.31 in 1999 to 5.70 in 2012 with the annual percentage change (APC) of 5.4 %. Five-year relative survival rate (RS) for myeloid malignancies has gradually improved for decades. RS changed from 26.3% to 34.8% in AML, specifically from 51.6% to 69.6% in acute promyelocytic leukemia (APL) and from 23.8% to 29.9% in non-APL AML, between 1996-2000 and 2008-2012. RS also increased from 81.8% to 87.1% in MPN, with a significant improvement in CML (from 74.5% to 85.5%), and from 27.3% to 31.7% in MDS/MPN between 2001-2005 and 2008-2012. However, there was no survival improvement in MDS during the study period (45.6% in 2001-2005 to 44.4% in 2008-2012).
Hematologic malignancies are classified on the basis of the morphology, immunophenotype, cytogenetics, and clinical characteristics. The most recent classification suggested by the World Health Organization (WHO) have grouped myeloid malignancies into five categories: acute myeloid leukemia (AML), myelodysplastic syndromes (MDS), myeloproliferative neoplasms (MPN), myelodysplastic and myeloproliferative (MDS/MPN) neoplasms, and other rare diseases associated with eosinophilia and abnormalities of growth factor receptors derived from platelets or fibroblasts [12]. The classification of these diseases has been modified to include recent advances in cytogenetics and molecular genetics for hematologic malignancies.
In cancer registries, the International Classification of Diseases for Oncology (ICD-O) has been widely used since 1976, for coding the tumor site and histology. The most recent publication of ICD-O is the third edition (ICD-O-3), published in 2000 and revised in 2013 [3]. This updated version of ICD-O-3 includes the new terms and code changes suggested by the updated WHO classification on tumors of hematopoietic and lymphoid tissues in 2008. Recent cancer registry reports adopted this ICD-O-3 classification, as it was considered to better reflect our current understanding of diseases [456].
In Korea, the Ministry of Health and Welfare started the Korea Central Cancer Registry (KCCR), a nationwide hospital-based cancer registry in 1980. The KCCR expanded to include the entire population under the population-based cancer registry program since 1999 [7]. ICD-O-3 was applied to all incident cases for neoplastic diseases since the year 2003.
The KCCR and the Korean Society of Hematology (KSH) reported the first nationwide statistics of hematologic malignancies 4 years ago, covering the incident cases from 1999 to 2008 [8]. In the previous analysis, disease entities were defined based on the International Classification of Diseases 10th edition (ICD-10) and calculated incidence and survival according to broad disease groups such as non-Hodgkin lymphoma (C82-C85, C96), myeloid leukemia (C92-C94), and lymphoid leukemia (C91).
The main objective of the current study is to update the statistical data on hematologic malignancies, focusing on myeloid malignancies, with the most recent database of KCCR in 2012. Incidence and survival estimates were analyzed according to more detailed disease groups compared to the previous report, using the ICD-O-3 codes. We also investigated recent epidemiologic changes of myeloid malignancies for decades in Korea with database from 1999 to 2012.
Incident cases of myeloid malignancies between 1999 and 2012 were obtained from the Korean National Cancer Incidence Database (KNCIDB) [9]. Myeloid malignancies were defined according to the revised version of ICD-O-3 (2013) and each code was categorized as shown in Supplementary Table 1, taking into account the clinical relevance. Myeloid malignancies were grouped into five categories: AML, MPN, MDS, MDS/MPN, and unknown myeloid neoplasms. AML and MPN were further divided into two subgroups as shown in Table 1, in consideration with acute promyelocytic leukemia (APL) and chronic myelogenous leukemia (CML), those have unique characteristics, treatments, and clinical outcomes separated from non-APL AML and non-CML MPN. For MPN, MDS, and MDS/MPN, incident cases between 1999 and 2002 were not included in this analysis because official registration by KCCR employing ICD-O-3 began in 2003. Codes for these categories by ICD-O-3 had significantly changed, but it could not be converted from old data.
Crude incidence rates (CRs) and age-specific incidence rates of each myeloid malignancy were calculated. The CRs per 100,000, an incidence rate based on the frequency of cancer in the entire population, were calculated by dividing the total number of events (N) by the total number of person-year of observation (P) and multiplying the result by 100,000. The age-specific incidence rates per 100,000 within age group i, were calculated by dividing the number of incidence observed in the age group (Ni) by the number of corresponding person-year of observation (Pi) and multiplying the result by 100,000. Age-standardized incidence rates (ASRs), weighted average of crude age-specific rates, were determined by using the mid-year population in Korea in 2000 as the standard population. Both age-specific incidence rates and ASRs were calculated according to age group: <14, 15-34, 35-49, 50-64, 65-79, and ≥80 years. Changes in the annual ASRs were examined by calculating the annual percentage change (APC) over a time period as (exp(b)-1)×100, where b is the slope of the regression of log(ASR) on a calendar year using the following linear regression equation [10]:
For the survival analysis, five-year relative survival rates were calculated. To find any changes for recent decades, KNCIDB data from 1996 to 2012 were included. The survival status of these patients was followed until December 31, 2013. Five-year relative survival (RS) rates for AML, and unknown myeloid neoplasms were calculated according to four periods of diagnosis: 1996-2000, 2001-2005, 2006-2010, and 2008-2012. RS for MPN, MDS, and MDS/MPN were calculated according to three periods of diagnosis (2001-2005, 2006-2010, and 2008-2012) since data before 2003 for these entities were not reliable. RS rates were developed by comparing the observed survival in the cancer patient group with the expected survival of the general population [11]. Five-year RS rates using the Ederer II method were based on an algorithm written by Paul Dickman in SAS [1213]. All analyses were performed using SAS version 9.2.
The overall number of incident cases for all neoplastic diseases in 2012 was 224,177. Myeloid malignancies occurred in 3,771 patients, which was 1.7% of all cancers (Fig. 1). It occurred in 2,138 men and 1,633 women, with a ratio of 1.3:1. Among all myeloid malignancies, AML (33.3%) was the most frequent, followed by MPN (33.8%), and MDS (21.6%). There was no significant difference between men and women.
Crude rates and age-specific incidence of myeloid malignancies by age group in 2012 were shown in Fig. 2 and 3, respectively. Patients with ages between 70 and 79 showed the highest CR of myeloid malignancies followed by ages 60 to 69, and ages 50 to 59. AML was most prevalent in patients who were less than 49 years old and those aged 60 to 69, while MPN and MDS were most prevalent in patients 50 to 59 years old and older than 70 years, respectively (Fig. 2). For age-specific incidence rates in 2012, AML was the most common myeloid disease in men patients younger than 50 years old. However, MDS surpassed AML in elderly patients with an age of 65 or more. In women, AML showed the highest incidence rate in patients up to 34 years old, and MPN substituted AML in ages above 35 years (Fig. 3).
The incident cases of myeloid malignancies and trend in CR and ASR between 1999 and 2012 was shown briefly in Table 1 (for detailed data, see Supplementary Table 2). During the study period, 36,924 cases of myeloid malignancies occurred. The overall ASR of all myeloid malignancies increased from 2.7 in 1999 to 5.7 in 2012. The APC was 7.4% between 1999 and 2012, which was higher than the APC of 3.5% for all cancers, and it was statistically significant. The ASRs increased from 1.88 to 2.02 in AML (APC=1.0%, P<0.05), from 1.70 to 1.76 in non-APL AML (APC=0.6%, not significant), from 0.18 to 0.27 in APL (APC=5.2%, P<0.05) from 1999 to 2012. The ASRs in MPN also increased from 1.34 to 1.95 (APC=4.7%, P<0.05), from 0.64 to 0.76 in CML (APC=1.7%, P<0.05), from 0.69 to 1.19 in non-CML MPN (APC=7.1%, P<0.05), from 2003 to 2012. Similar increasing trends were observed both in MDS and MDS/MPN, from 0.76 to 1.13 in MDS (APC=5.8%, P<0.05) and from 0.06 to 0.12 in MDS/MPN (APC=9.3%, P<0.05) between 2003 and 2012, respectively. The number of unknown myeloid neoplasms had decreased, but still counted up to 341 cases in 2012.
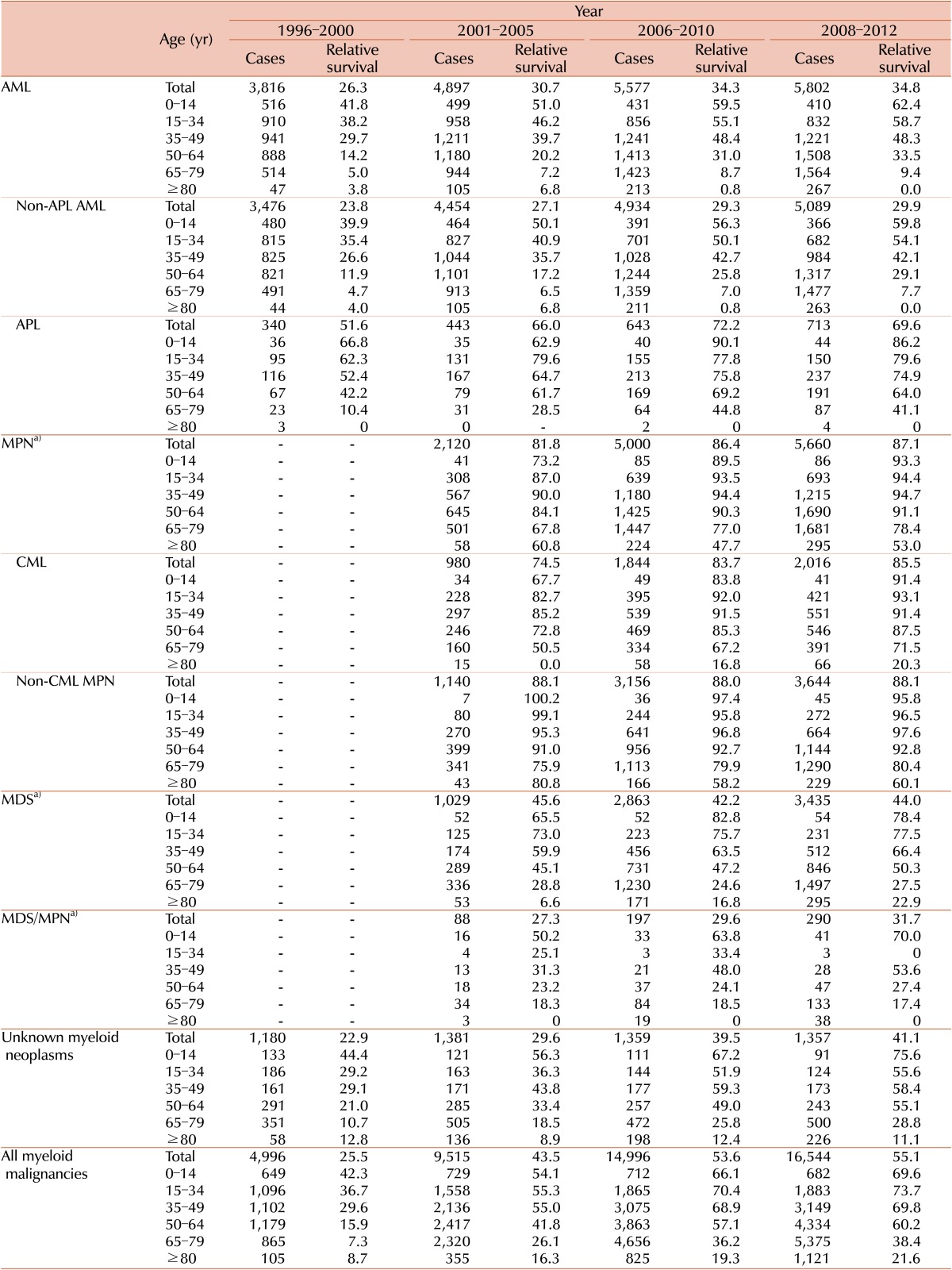
Five-year RS rates for patients with myeloid malignancies for four intervals (1996-2000, 2001-2005, 2006-2010, and 2008-2012) stratified by years and age are described in Table 2. RS varied between the disease entities: being 34.8% for AML (29.9 % for non-APL AML and 69.6% for APL), 87.1% for MPN (85.5% for CML and 88.1% for non-CML MPN), 44% for MDS, and 31.7% for MDS/MPN in 2008-2012.
More detailed survival data for subcategories are described in Supplementary Table 3. Polycythemia vera showed the most favorable outcome with a 5-year relative survival rate of 99.7%, followed by essential thrombocythemia with a rate of 90.3%. Poor survival in some disease entities, such as AML with multilineage dysplasia or transformed AML from MDS (17.8%), acute panmyelosis with fibrosis (13.9%), MDS-refractory anemia with excess blasts (24.3%), and chronic myelomonocytic leukemia (23.2%), was noted. In most cases, the RS of the patients with myeloid malignancies decreased as their age increased.
Five-year RS for all myeloid malignancies has gradually improved recently, from 25.5% in 1996-2000 to 55.1% in 2008-2012, with an increase of 29.6%. For last few decades, survival rates for most myeloid malignancies improved except for non-CML MPN, and MDS. Specifically, 5-year RS rates changed from 26.3% to 34.8% in AML from 1996-2000 to 2008-2012 (from 23.8% to 29.9% in non-APL AML, and 51.6% to 69.6% in APL). The 5-year RS rates increased from 81.8% to 87.1% in MPN, with significant improvement in CML (from 74.5% to 85.5%), from 2001-2005 to 2008-2012. Survival in MDS/MPN also increased from 27.3% to 31.7% from 2001-2005 to 2008-2012. However, there was no improvement of relative survival in MDS during the study period (45.6% in 2001-2005 to 44.4% in 2008-2012). Changes in survival curves for myeloid diseases are shown in Fig. 4.
We showed the epidemiologic data for myeloid malignancies in Korea between 1996 and 2012, updating the previous report [8], which had focused on analyzing the basic characteristics and past survival rates of domestic hematologic malignancies roughly classified according to ICD-10 code [14]. In this study, we adopted the latest ICD-O-3 codes [3] and the WHO classifications [1] to distinguish many different subcategories based on the cell lineage, histologic/genetic characteristics, and clinical prognosis, to avoid grouping clinically different diseases together as a single vague entity such as 'myeloid leukemia'. Analysis with subcategories enriched this study with data closely related to real-life practice, although it caused some artifacts in survival estimates owing to the small number of rare diseases.
Calculating population-based relative survival can measure survival improvement in each myeloid malignancy, which can be more easy compared to conventional cohort approaches or clinical trials. In Korea, the KNCIDB includes entire annual population diagnosed with malignant diseases, which is enough to truly represent the nationwide status.
Consistent with the first report by KCCR [8], we found an increasing incidence of most myeloid malignancies by age and year at diagnosis. Furthermore, increasing incidences in MDS, MPN, MDS/MPN were also detected, which could not be calculated in the previous report. Age and environmental factors [151617] can be considered a potential cause of inflated incidence. A similar change was observed in the European HAEMACARE project [18], but Korean ASRs of MDS and MPN are much lower than that of European, probably because of ethnic or regional differences. The increased number of cancer survivors owing to the improvements in overall cancer survival [19] also needs to be considered because the risk of myeloid malignancies increases after chemoor radiotherapy [20], although the exact effect would be difficult to validate.
The relative survival for most myeloid malignancies was comparable to other country. Five-year survival of patients with AML (non-APL AML) was 29.9% in 2008-2012 and this is better than or comparable to European data (15% in 2006-2008) [21] and US data (25.9% in 2005-2011) [6]. Because the conventional chemotherapy with anthracycline and cytarabine has been widely applied for AML without significant change for years, prolonged survival may be due to the improvement in transplantation and supportive care such as anti-infective agents and immunosuppressive agents [222324]. Best supportive care might influence on reduced early mortality in APL, with an introduction of arsenic trioxide for relapsed disease or frail patients to anthracycline.
For MDS, relative survival was significantly different between the subtypes, ranging from 24.3% for refractory anemia with excess blasts to 68.1% for MDS with 5q deletion. This is the first time that survival analysis according to the subtypes of MDS was performed.
Poor survival of patients with AML and high-risk MDS was more apparent in patients over 65 years old. It is well known that elderly AML or MDS patients are associated with unfavorable tumor biology compared to younger patients, but they also tend to be too frail to undergo intensive treatments, including hematopoietic stem cell transplantation required to induce the potential cure [2526]. Survival improvements in elderly AML and MDS were not apparent compared to younger patients so far. In Korea, hypomethylating agents were approved for elderly patients with AML [27]. We should find the effect of this noble treatment in the future analysis
In the presented data, relative survival of CML strikingly increased in the 2000s, and became more apparent in the late 2000s. The main cause of such improvement is attributed to the introduction of tyrosine kinase inhibitors, as expected. In Korea, imatinib, the first tyrosine kinase inhibitor, was introduced in 2001 as a phase III clinical trial for approval. After its formal approval in 2006, this effective treatment has been prescribed widely. Five-year relative survival for patients with CML in our data (69.4% in 2001-2005 and 83.7% in 2006-2010) seems to be higher than in data from western countries (SEER database: 63.2% in 2005-2011 and EUROCARE data: 46% in 2003-2005) [621], although direct comparison between the databases has many limitations.
In terms of unknown myeloid malignancies, acute leukemia with ambiguous lineage and other vague entities (leukemia, acute leukemia, and myeloid leukemia) that cannot be classified to any categories of the WHO classification. The proportion of cases reported with vague terms among all myeloid malignancies has decreased from 24.2% in 1999 to 8.6% in 2012. To reduce this proportion further, we should make an effort to diagnose the disease using exact terms and codes. Annual education and update of recent changes in classification of hematologic diseases should be provided for medical record administrators.
In summary, we found an increasing incidence according to age and year at diagnosis for most myeloid malignancies. Patient survival was also improved over the study periods, mainly because of a notable increase in survival of patients with APL and CML owing to the widespread use of more effective and less toxic treatments and supportive care. Serial reports of population-based cancer registry data enable us to monitor the improvements in survival associated with recent advances in diagnosis and treatment for hematologic malignancies. To perform this essential task, maintaining the quality of cancer registries through comprehensive data collection and monitoring, adequate education programs, and recruiting stable investment for resources are prerequisites. A qualified registry would provide more amount of informative evidence as to whether the recent changes in treatment do lead to improved survival.
References
1. Swerdlow SH, Campo E, Harris NL, editors. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon, France: IARC Press;2008.
2. Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009; 114:937–951. PMID: 19357394.

3. Percy CL, Fritz AG, Jack A, editors. International classification of diseases for oncology (ICD-O). 3rd ed. Geneva, Switzerland: World Health Organization;2013.
4. Marcos-Gragera R, Allemani C, Tereanu C, et al. Survival of European patients diagnosed with lymphoid neoplasms in 2000-2002: results of the HAEMACARE project. Haematologica. 2011; 96:720–728. PMID: 21330324.

5. Sant M, Allemani C, Tereanu C, et al. Incidence of hematologic malignancies in Europe by morphologic subtype: results of the HAEMACARE project. Blood. 2010; 116:3724–3734. PMID: 20664057.

6. National Cancer Institute. Surveillance, Epidemiology, and End Results (SEER) Program Research Data (1973-2012). Bethesda, MD: National Cancer Institute;2015. Accessed October 2, 2015. http://seer.cancer.gov/data/.
7. Shin HR, Won YJ, Jung KW, et al. Nationwide cancer incidence in Korea, 1999~2001; First result using the national cancer incidence database. Cancer Res Treat. 2005; 37:325–331. PMID: 19956367.

8. Park HJ, Park EH, Jung KW, et al. Statistics of hematologic malignancies in Korea: incidence, prevalence and survival rates from 1999 to 2008. Korean J Hematol. 2012; 47:28–38. PMID: 22479275.

9. Korea Central Cancer Registry, National Cancer Center. Annual report of cancer statistics in Korea in 2012. Seoul, Korea: Ministry of Health and Welfare;2014.
10. Howlader N, Noone AM, Krapcho M, et al. SEER cancer statistics review, 1975-2010. Bethesda, MD: National Cancer Institute;2015. Accessed October 2, 2015. http://seer.cancer.gov/csr/1975_2010/.
11. Ederer F, Axtell LM, Cutler SJ. The relative survival rate: a statistical methodology. Natl Cancer Inst Monogr. 1961; 6:101–121. PMID: 13889176.
12. Ederer F, Heise H. Instructions to IBM 650 programmers in processing survival computations, technical, end results evaluation section. Bethesda, MD: National Cancer Institute;1959.
13. Dickman PW, Sloggett A, Hills M, Hakulinen T. Regression models for relative survival. Stat Med. 2004; 23:51–64. PMID: 14695639.

14. World Health Organization. International statistical classification of diseases and related health problems (ICD-10) in occupational health. Geneva, Switzerland: World Health Organization;1999.
16. Alexander FE. The search for causes of the leukaemias. Eur J Cancer. 1995; 31A:863–867. PMID: 7646912.

17. Gorini G, Stagnaro E, Fontana V, et al. Alcohol consumption and risk of leukemia: A multicenter case-control study. Leuk Res. 2007; 31:379–386. PMID: 16919329.

18. Maynadié M, De Angelis R, Marcos-Gragera R, et al. Survival of European patients diagnosed with myeloid malignancies: a HAEMACARE study. Haematologica. 2013; 98:230–238. PMID: 22983589.

19. Jung KW, Won YJ, Kong HJ, Oh CM, Lee DH, Lee JS. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2011. Cancer Res Treat. 2014; 46:109–123. PMID: 24851102.

21. Sant M, Minicozzi P, Mounier M, et al. Survival for haematological malignancies in Europe between 1997 and 2008 by region and age: results of EUROCARE-5, a population-based study. Lancet Oncol. 2014; 15:931–942. PMID: 25030467.

22. Gooley TA, Chien JW, Pergam SA, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. 2010; 363:2091–2101. PMID: 21105791.

23. Higby DJ, Cohen E, Holland JF, Sinks L. The prophylactic treatment of thrombocytopenic leukemic patients with platelets: a double blind study. Transfusion. 1974; 14:440–446. PMID: 4607226.

24. Walsh TJ, Finberg RW, Arndt C, et al. National Institute of Allergy and Infectious Diseases Mycoses Study Group. Liposomal amphotericin B for empirical therapy in patients with persistent fever and neutropenia. National Institute of Allergy and Infectious Diseases Mycoses Study Group. N Engl J Med. 1999; 340:764–771. PMID: 10072411.
25. Appelbaum FR, Gundacker H, Head DR, et al. Age and acute myeloid leukemia. Blood. 2006; 107:3481–3485. PMID: 16455952.

26. Kantarjian H, O'brien S, Cortes J, et al. Results of intensive chemotherapy in 998 patients age 65 years or older with acute myeloid leukemia or high-risk myelodysplastic syndrome: predictive prognostic models for outcome. Cancer. 2006; 106:1090–1098. PMID: 16435386.
27. Medeiros BC, Satram-Hoang S, Hurst D, Hoang KQ, Momin F, Reyes C. Big data analysis of treatment patterns and outcomes among elderly acute myeloid leukemia patients in the United States. Ann Hematol. 2015; 94:1127–1138. PMID: 25791241.

Supplementary Materials
Supplementary Table 2
Incident cases of myeloid malignancies and trend in crude incidence rates and age-standardized incidence rates in Korea from 1999 to 2012 (in detail).
Supplementary Table 3
Five-year relative survival rates of myeloid malignancies by age group in Korea (in detail).
Fig. 1
Incident cases of myeloid malignancies in men (B) and women (C) in Korea, 2012. a)AML cases include 153 cases of APL in both genders (77 cases in men, and 76 cases in women); b)MPN cases include 458 cases of CML in both genders (288 cases in men, and 170 cases in women); c)Unknown cases include 15 cases of acute leukemia, ambiguous lineage in both genders (7 cases in men, 8 cases in women).
Abbreviations: AML, acute myeloid leukemia; MPN, myeloproliferative neoplasms; MDS, myelodysplastic syndrome; MDS/MPN, myelodysplastic/myeloproliferative neoplasms.
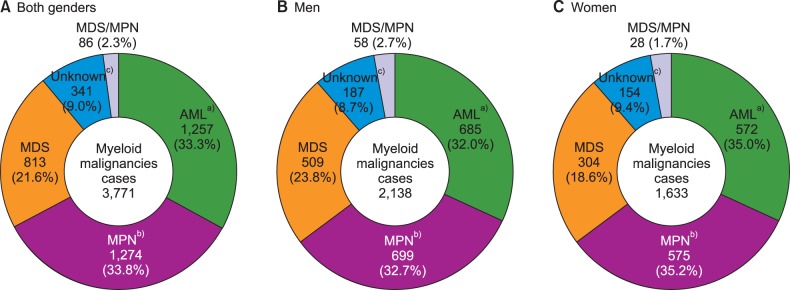
Fig. 2
Incident cases of myeloid malignancies by age group in Korea, 2012.
Abbreviations: same as in Fig. 1.
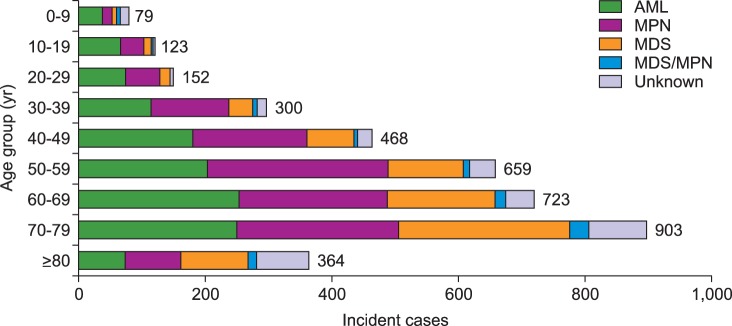
Fig. 3
Age-specific incidence rates of myeloid malignancies in men (A) and in women (B) in Korea, 2012.
Abbreviations: same as in Fig. 1.
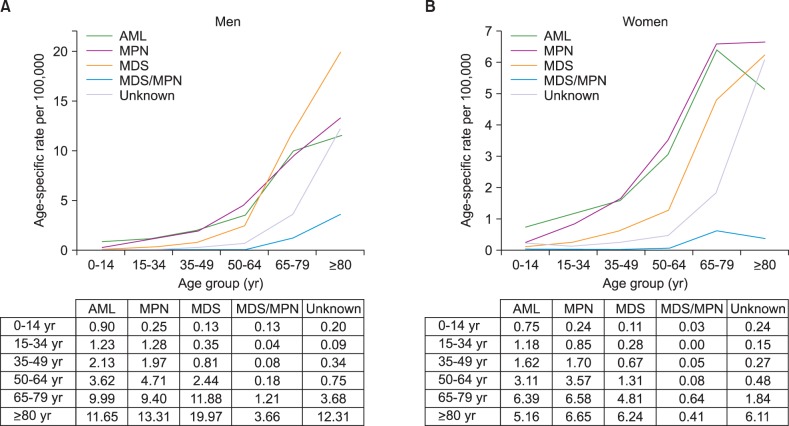
Fig. 4
Trend in relative survival rate of myeloid malignances between 1996 and 2010 in Korea.
Abbreviations: same as in Fig. 1.
Table 1
Incident cases of myeloid malignancies and trend in crude incidence rates and age-standardized incidence rates in Korea from 1999 to 2012.
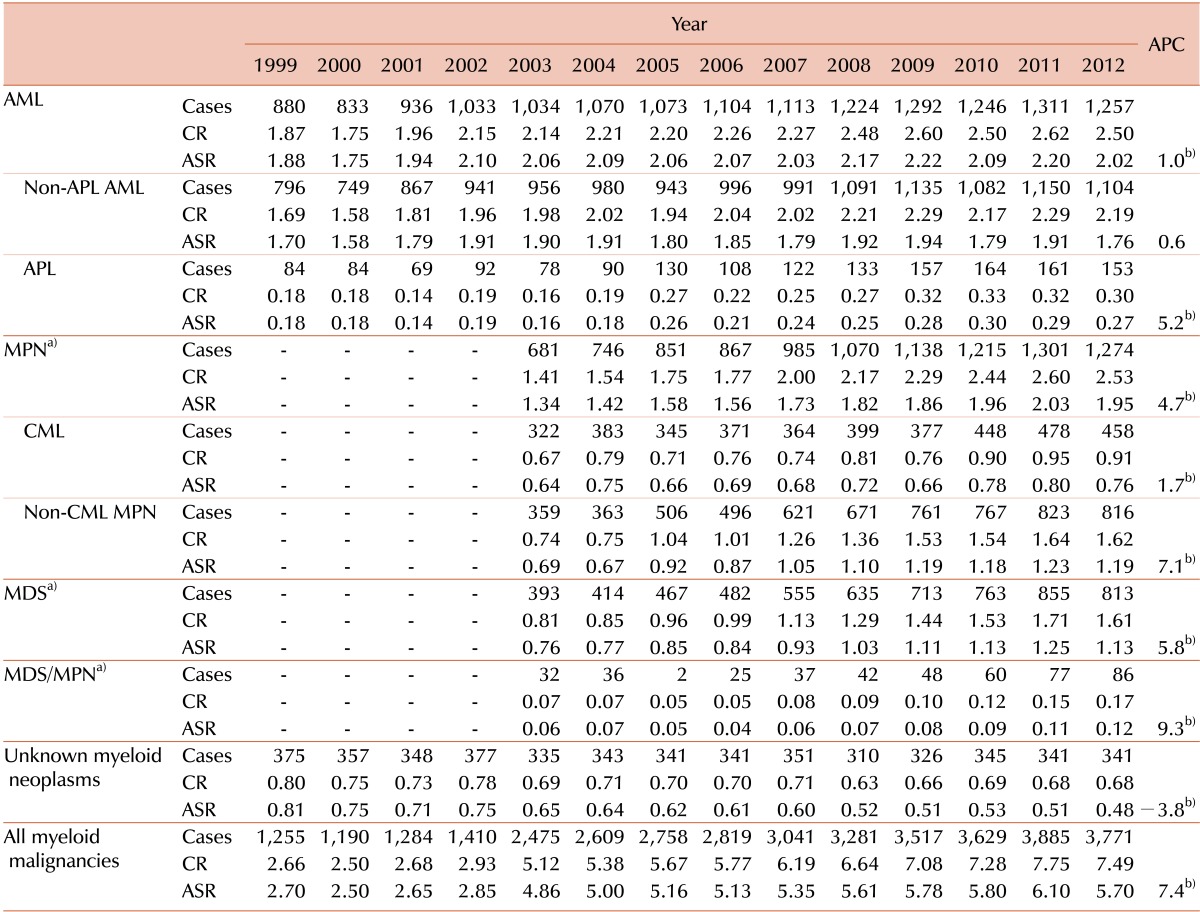
a)Official registration employing ICD-O-3 began in 2003 at KCCR. b)The annual percent change is statistically significantly different from zero (P<0.05).
Abbreviations: CR, crude incidence rate; ASR, age-standardized incidence rate; APC, annual percentage change; APL, acute promyelocytic leukemia; AML, acute myeloid leukemia; CML, chronic myelogenous leukemia; MPN, myeloproliferative neoplasia; MDS, myelodysplastic syndrome.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download