TO THE EDITOR: Thymoma is known to be associated with autoimmune disorders such as myasthenia gravis, which occurs in approximately 30% of cases [1], and sometimes is also accompanied by T-cell lymphocytosis [2]. However, thymoma patients with concurrent myasthenia gravis rarely develop lymphocytosis [3]. In the majority of cases, thymoma-associated lymphocytosis consists of a polyclonal population of mature T cells expressing CD4, CD8, or neither marker [2]. We report the unusual case of a patient with a history of invasive thymoma and myasthenia gravis who was subsequently found to have peripheral blood (PB) and bone marrow (BM) CD8+ T-cell lymphocytosis and pure red cell aplasia.
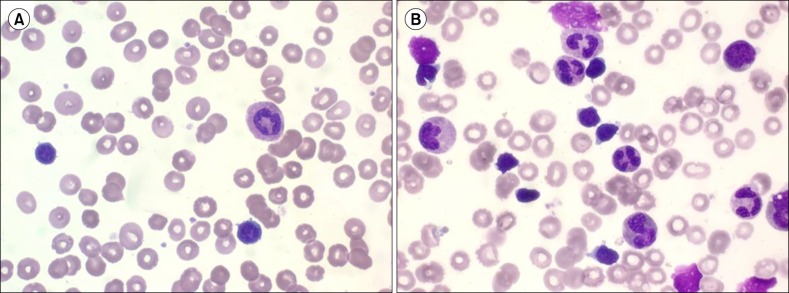
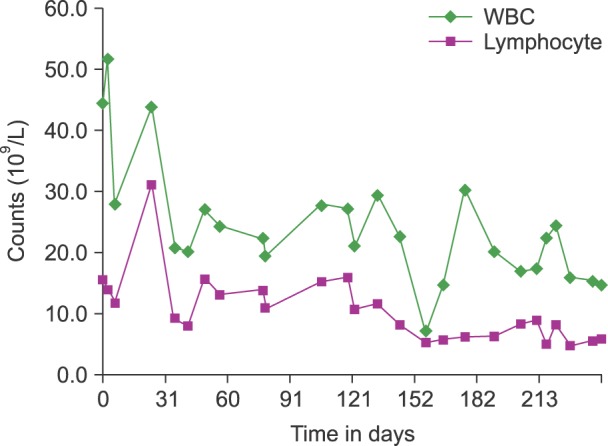
A 38-year-old woman presented to the emergency department with abdominal pain. Nine years earlier, she had been diagnosed as having invasive thymoma and myasthenia gravis. The thymoma had been treated surgically, followed by chemotherapy and radiation therapy until one year prior to the present admission. A computed tomography scan of her abdomen and chest revealed multiple thymoma metastases involving the lung, pleura, pericardium, liver, both ovaries, and peritoneal seeding. Percutaneous needle biopsy of the pleural mass was consistent with thymoma. The patient's complete blood count showed the following results: white blood cell count: 44.4×109/L (58.9% neutrophils, 34.9% lymphocytes); hemoglobin: 9.5 g/dL; platelet count: 170×109/L. In the workup for anemia, progressive anemia (6.7 g/dL) and persistent lymphocytosis (7.8-31.0×109/L) were noted over 3 months. Analysis of a PB smear showed an increased number of mature lymphocytes with dense chromatin, no nucleoli, and scanty agranular cytoplasm (Fig. 1). Flow cytometry of the PB revealed that 78.4% of lymphocytes were CD3+ and CD8+ T cells. The ratio of CD4+/CD8+ cells was 0.13. A BM study revealed mild hypocellular marrow with erythroid aplasia and diffuse scattered infiltration of small lymphocytes (26.3% lymphocytes on BM aspirate) (Fig. 1). Immunophenotyping by flow cytometry showed proliferation of T cells with expression of CD2, CD3, CD5, CD7, and CD8, which was consistent with the pattern expressed by the T cells in the PB. The result of TCR gene rearrangement in the BM demonstrated no clonal rearrangement. The patient underwent one cycle of chemotherapy with cisplatin, etoposide, and ifosfamide to treat thymoma. After the first cycle, chemotherapy was put on hold because the patient was in poor clinical condition. PB analysis showed somewhat reduced T-cell lymphocytosis (5.8×109/L) over four months of observation (Fig. 2).
To the best of our knowledge, this is the first case report of PB and BM CD8+ T-cell lymphocytosis occurring concurrently with invasive thymoma. In most reported cases of T-cell lymphocytosis associated with thymoma, lymphocytes consist of a mixed mature population of T cells with a normal or slightly abnormal CD4:CD8 ratio [2456]. In the present case, besides peripheral T-cell lymphocytosis, a predominance of mature lymphocytes was found in the BM, which primarily raised the suspicion of T-cell leukemia/lymphoma. Chronic peripheral T-cell lymphocytosis with BM lymphocytosis generally reflects proliferation of a neoplastic T-cell clone. The differential diagnosis between reactive lymphocytosis and lymphoma/leukemia is of chief importance in patients with thymoma [2]. Flow cytometric studies and TCR gene rearrangement analysis can establish the monoclonality or polyclonality of lymphocytes, therefore resulting in the appropriate diagnosis. The current patient was diagnosed with T-cell lymphocytosis associated with thymoma, which was confirmed with a combination of lymphocyte morphology, flow cytometric studies, and TCR gene rearrangement.
The etiology of T-cell lymphocytosis associated with thymoma is uncertain. It has been speculated that lymphocytosis can be caused by the 'spillover' effect of thymic lymphocytes into the PB [7]. Other theories such as thymoma-related dysregulation of BM hematopoiesis and 'ill-defined immunoregulatory disorder' based on thymic hormone imbalances have been proposed [28]. Thymoma is the only tumor proven to generate mature T cells from immature precursors. It has been reported that the proportion of circulating CD45RA+ CD8+ T cells is significantly increased in patients with thymoma compared with that in normal controls, and this phenomenon is due to their export from the tumor itself [9]. The increase of CD8+ T cells with significantly decreased CD4/CD8 ratio found in our patient was in agreement with that finding.
In our case, PB and BM lymphocytosis was an unusual CD8+ T-cell lymphocytosis. The presence of a relatively mature, persistent CD8+ T cell expansion in the absence of molecular evidence of monoclonal proliferation should provide a strong clue for the diagnosis of thymoma associated T cell lymphocytosis.
References
1. Morgenthaler TI, Brown LR, Colby TV, Harper CM Jr, Coles DT. Thymoma. Mayo Clin Proc. 1993; 68:1110–1123. PMID: 8231276.

2. Barton AD. T-cell lymphocytosis associated with lymphocyte-rich thymoma. Cancer. 1997; 80:1409–1417. PMID: 9338464.

3. Bauduer F, Archambaud F, Mazères F, Ellie E, Ducout L. T-cell lymphocytosis associated with thymoma and myasthenia. Rev Med Interne. 2002; 23:951–952. PMID: 12481398.
4. Morales M, Trujillo M, del Carmen Maeso M, Piris MA. Thymoma and progressive T-cell lymphocytosis. Ann Oncol. 2007; 18:603–604. PMID: 17074971.

5. Puljiz Z, Karin Z, Bratanic A, et al. Late distant metastases of malignant thymoma associated with peripheral T-cell lymphocytosis. Pathol Int. 2013; 63:516–518. PMID: 24147431.

6. Chen HK, Huang WT, Eng HL, Lu HI, Huang HY. Ossifying thymoma clinically presenting with peripheral T-cell lymphocytosis. Ann Thorac Surg. 2009; 88:e5–e7. PMID: 19559177.

7. Pedraza MA. Thymoma immunological and ultrastructural characterization. Cancer. 1977; 39:1455–1461. PMID: 300647.

8. Medeiros LJ, Bhagat SK, Naylor P, Fowler D, Jaffe ES, Stetler-Stevenson M. Malignant thymoma associated with T-cell lymphocytosis. A case report with immunophenotypic and gene rearrangement analysis. Arch Pathol Lab Med. 1993; 117:279–283. PMID: 8382915.
9. Hoffacker V, Schultz A, Tiesinga JJ, et al. Thymomas alter the T-cell subset composition in the blood: a potential mechanism for thymoma-associated autoimmune disease. Blood. 2000; 96:3872–3879. PMID: 11090072.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download