Abstract
Background
Inhibitory antibodies to factor VIII (FVIII) or IX (FIX) are important issues when managing patients with hemophilia A or B. Advances in bypassing agents such as recombinant activated FVII (rFVIIa) and activated prothrombin complex concentrates (APCC) have enabled the aggressive management of hemophilia with inhibitors during emergency or elective surgery. This study provides an updated evaluation of the safety and effectiveness of bypassing agents in treating perioperative bleeding.
Methods
We reviewed the records of hemophilia patients with inhibitors who underwent surgery between May 2008 and July 2014 using bypassing agents or high-dose FVIII concentrates at a single center.
Results
In total, 36 surgeries (24 orthopedic, 12 other) were conducted in 18 hemophilia patients with inhibitors. The median inhibitor titer at surgery was 14 (range, 0.7-1,900) Bethesda units. Most patients had high-responding inhibitors. In total, 25 patients received APCC, 9 with rFVIIa initially. In most cases, bleeding stopped or was well controlled; however, bleeding in 6 patients was controlled using sequential bypassing therapy. Hemostatic efficacy of bypassing agents in various surgeries, based on the final patient outcome, was 94.4% (34/36). Among 5 emergency surgeries, 2 deaths occurred.
Conclusion
Good control of hemostasis can be achieved using bypassing agents in hemophilia patients with inhibitors who are undergoing surgery. Thorough planning is needed before elective surgery and more active and aggressive management may be needed for emergency surgery. Use of bypassing agents can facilitate safe and successful surgeries in hemophilia patients with inhibitors.
Go to : 
Inhibitory antibodies to factor VIII (FVIII) or IX (FIX) are formed as notable complications in 10-30% and 2-5% of patients with hemophilia A and B, respectively [1]. This poses challenging problems for clinicians treating patients with hemophilia A or B undergoing surgical interventions in that inhibitors not only rapidly inactivate coagulation factordeficient concentrates but also stimulate the synthesis of new antibodies [2]. According to a recent European study [3], hemophilia patients with inhibitors are more vulnerable to arthropathy and orthopedic and musculoskeletal complications leading to a prolonged hospital stay and uncontrolled bleeding compared with those without inhibitors. Additionally, hemophilia patients with inhibitors are vulnerable to acute and chronic diseases arising from recurrent bleeding episodes, for which they should undergo surgical corrections [4]. It is commonly noted that it is impossible to supplement deficient coagulation factors. It is thus important to formulate appropriate treatment plans for hemophilia patients with inhibitors who are planning to undergo surgery [5]. That is, prevention for bleeding should be considered during the perioperative period. Bypassing agents are used to control and prevent bleeding during the perioperative period in hemophilia patients with inhibitors [12678]. Two types of bypassing agents are currently available in the clinical setting: activated prothrombin complex concentrates (APCC) (FEIB; Baxter, Vienna, Austria) and recombinant activated factor VII (rFVIIa) (NovoSeven; Novo Nordisk, Bagsvaerd, Denmark). A few clinical studies have shown that bypassing agents can be safe, effective treatments to manage bleeding before and after surgery and to prevent bleeding in hemophilia patients with inhibitors [1267]. However, the number of reported cases involving emergency conditions and elective surgeries remains limited, and a consensus regarding the efficacy and safety of bypassing agents is still needed.
Given this background, we conducted this single-center, retrospective study to assess the hemostatic efficacy and safety of bypassing agents in hemophilia patients with inhibitors undergoing elective or emergency surgeries. The aim of this study was to identify the possibility of surgical intervention in hemophilia patients with inhibitors by using bypassing agents while under the care of hematologists.
Go to : 
Between May 2008 and July 2014, 18 patients underwent 36 surgeries at our medical institution. Hemophilia patients with inhibitors who underwent surgery and were hospitalized for hemostatic therapy were included. Inhibitors were classified into low- or high-responding inhibitors based on a patient's peak inhibitor titer after repeated FVIII exposure. An antibody titer persistently below 5 Bethesda units (BU) despite repeated challenges with FVIII was considered a low-responding inhibitor. A high-responding inhibitor was defined as a titer greater than 5 BU at any time [9]. This study was approved by the Institutional Review Board of our medical institution (approval No. 2015-01-028).
High-dose FVIII concentrates (100 IU/kg twice daily) were used in the low-responding inhibitor group. In the high-responding inhibitor group, bypassing agents were administered following the manufacturer's guidelines for optimal dosing: 50-100 U/kg for APCC and 90-120 µg/kg for rFVIIa [1011]. APCC was administered every 8-12 hours but did not exceed 200 IU/kg/day for the first 3 days [12]. The rFVIIa was administered every 2-3 hours in doses of 90 µg/kg for the first 3 days. The infusion regimens of bypassing agents were prescribed in accordance with a nationally approved summary of product characteristics or in accordance with guidance from current published literature [613]. The treatment dose was tapered depending on the type of surgery and clinical outcomes. If surgery was performed under general anesthesia or if the surgery was orthopedic, we reduced the dose or frequency of clotting factor concentrates after using the current product for the first 3 days. If the procedure was performed under local anesthesia, we controlled the use of hemostatic coverage after the first 2 days. Considering clinical outcomes, if the bleeding worsened or the patient did not improve, additional changes in treatment were made such as switching products or increasing the dose or frequency of the current product. Because the maximum dose of bypassing agent was administered at the beginning of the surgery, the agent was changed if there was unsatisfactory bleeding control.
If patients with hemophilia and inhibitors experienced bleeding episodes that were refractory to either APCC or rFVIIa alone, both products were administered in a sequential fashion to produce a superior hemostatic outcome. Sequential therapy was defined as the alternate administration of 1 APCC dose followed by 1 or 2 rFVIIa doses within 12 hours.
Through a retrospective review of the medical records, we evaluated preoperative baseline characteristics such as age, gender, weight, type and severity of hemophilia, inhibitor titers, medical history, type of surgery, type of initial bypassing agent, initial treatment regimens, and outcomes of prophylactic treatment. We also evaluated patient outcomes based on perioperative bleeding, time to change of initial clotting factor concentrates, use of sequential therapy, total amount of clotting factor concentrates, reoperation or additional surgery, length of hospital stay, and patient outcomes such as the rate of patient discharge.
Postoperatively, the patients were followed up once during the first 1-month period, at 6-month intervals for the following year, and once per year during the second and third years.
Go to : 
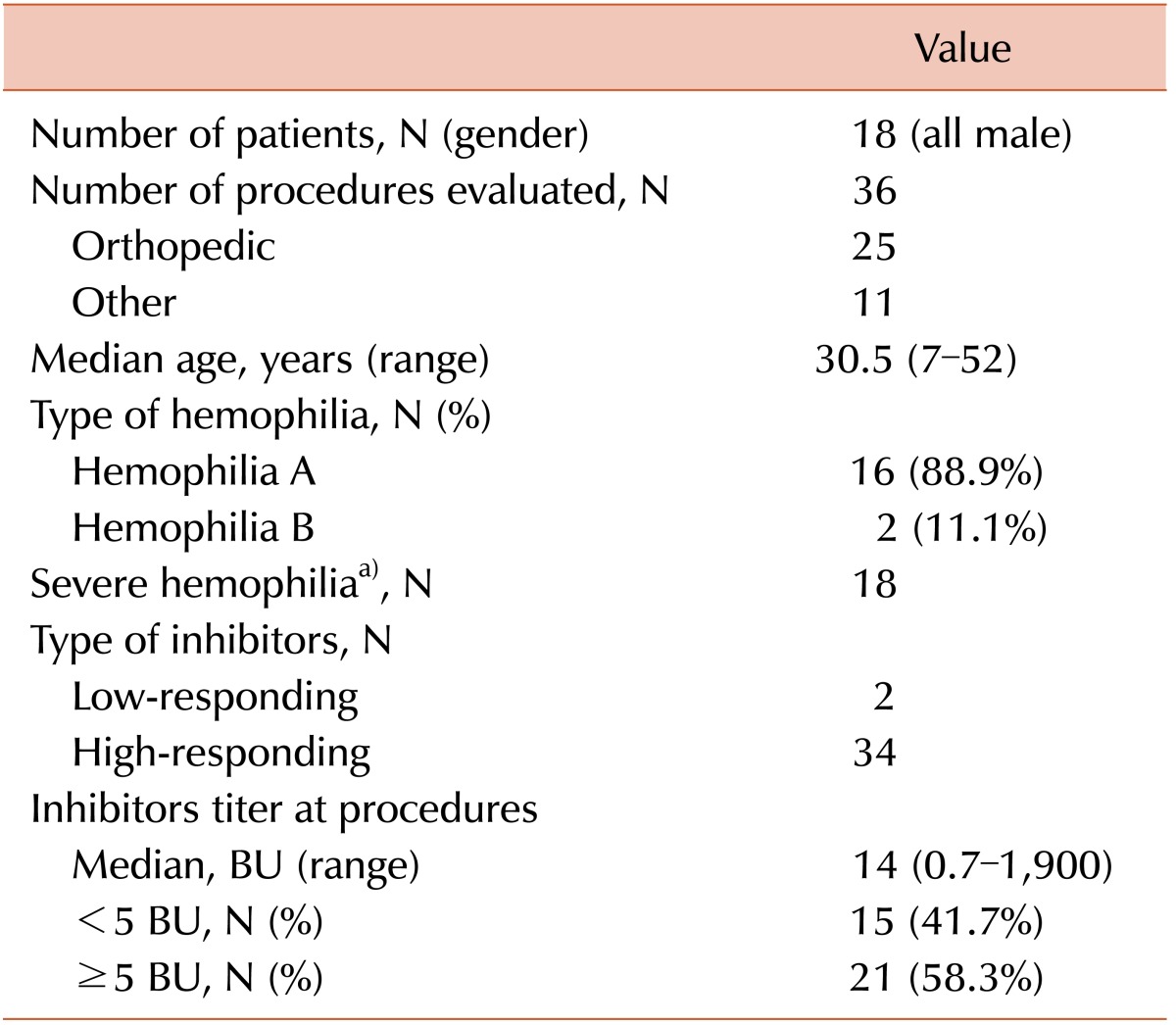
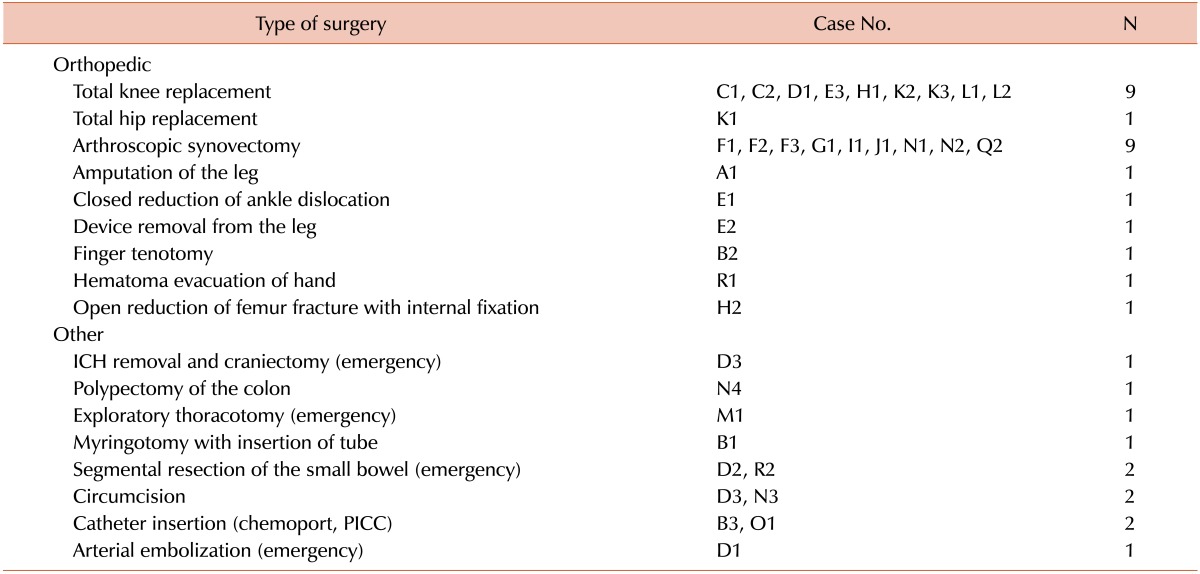
In total, 36 surgeries were conducted in 18 hemophilia patients with inhibitors (Table 1). The median age of the patients was 30.5 (range, 7-52) years. Our clinical series of patients comprised 16 cases of hemophilia A (88.9%) and 2 cases of hemophilia B (11.1%). All of the patients had severe hemophilia. which shows less than 1% of normal factor activity in blood. The median inhibitor titer at procedures was 14 (range, 0.7-1,900) BU. Of the 36 surgeries, 25 were elective orthopedic surgeries, and the remaining 11 comprised a variety of surgical procedures, 5 of which occurred under emergency conditions. All surgeries were performed using standard conventional methods at a single center, for which the patients' hematologic profile was monitored by a single board-certified specialist in hematology. Moreover, each procedure is reported as an individual case (Tables 2 and 3).
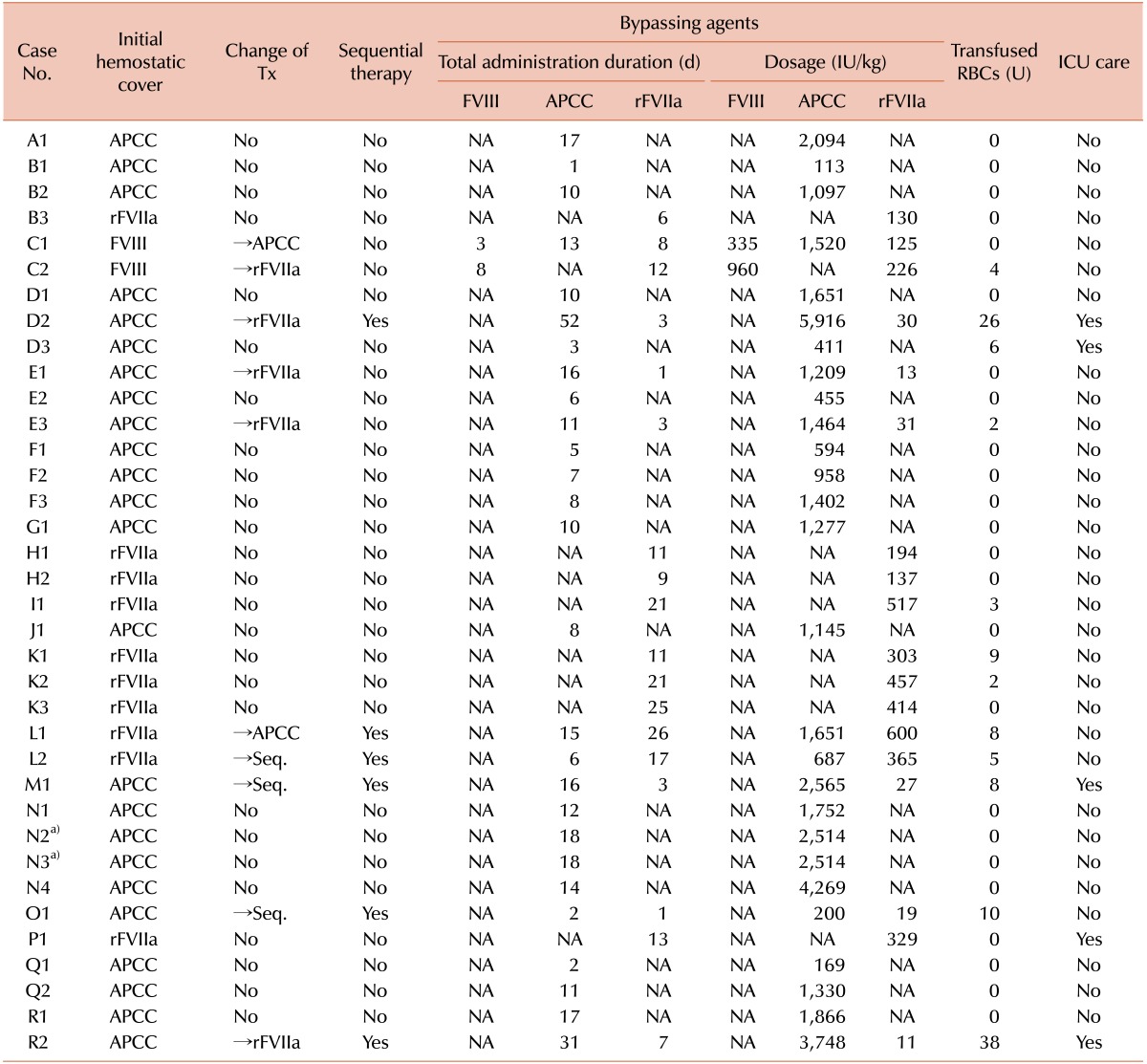
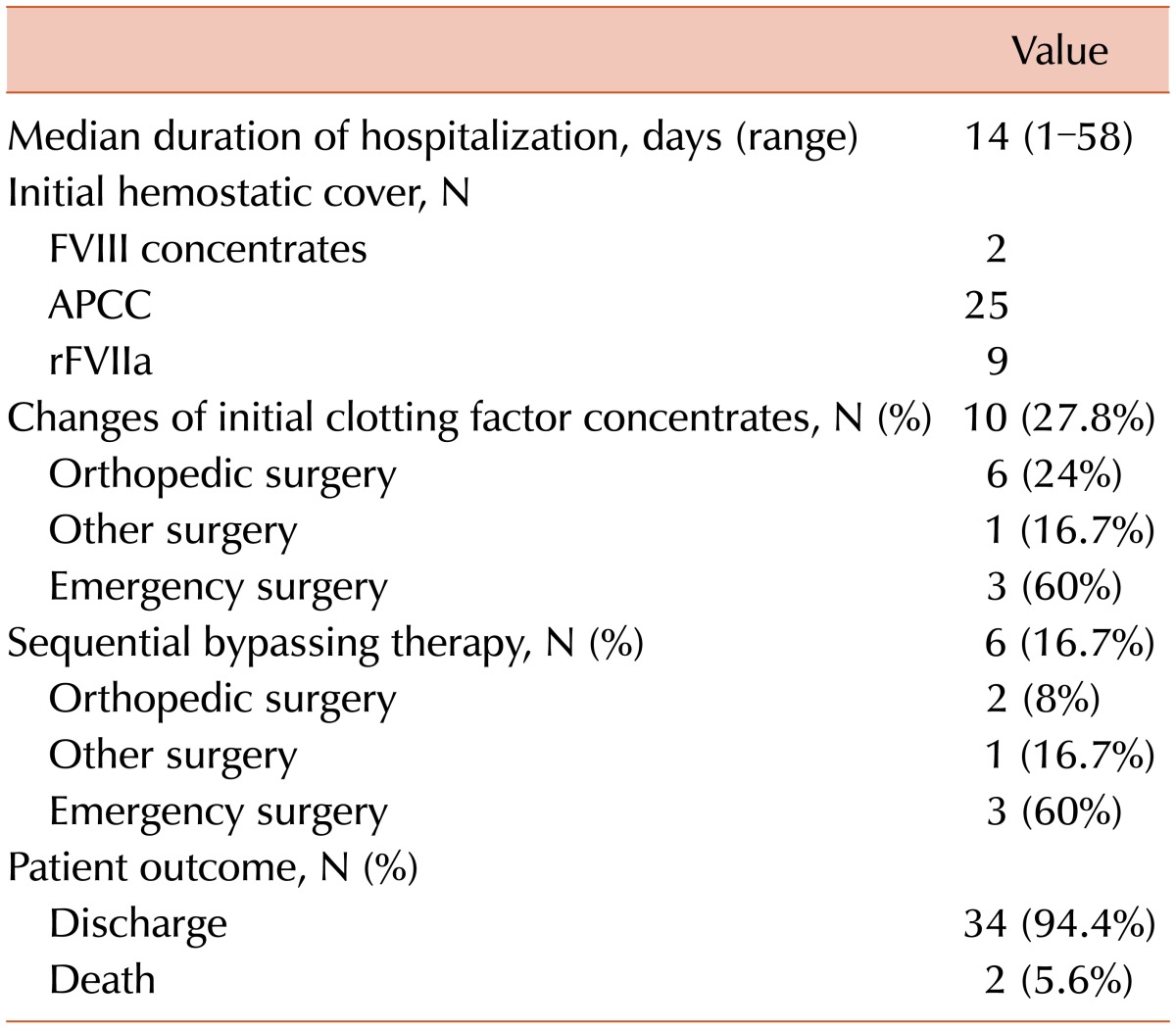
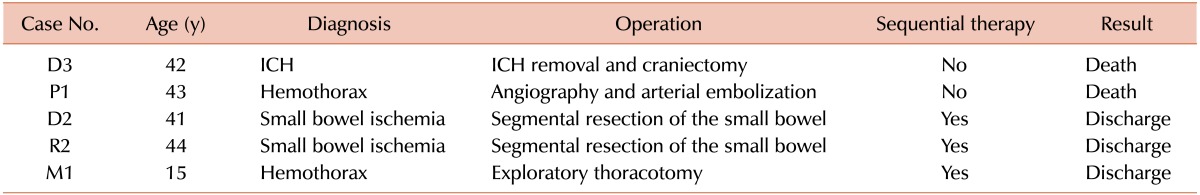
Patient progress and outcomes are described in Table 4. The median length of hospital stay was 14 (range, 1-58) days. High-dose FVIII concentrates were given in 2, APCC in 21, and rFVIIa in 9 cases (Table 1). In 10 cases, however, we replaced the initial hemostatic cover with other medications for continuous bleeding control. If satisfactory bleeding control was not achieved by changing the bypassing agent, sequential bypassing therapy was performed (case numbers D2, L1, L2, M1, O1, and R2). In these cases, bleeding control was successful within several days after sequential therapy with APCC and rFVIIa (Tables 2 and 4).
In our series, the rate of patient discharge was 94.4% (34/36). One patient (D2) had a hospital stay that was prolonged to 58 days for additional surgeries. This patient presented with gastrointestinal bleeding due to small bowel ischemia. Moreover, the patient developed shock due to continuous bleeding from the sites of anastomosis, for which the patient needed additional surgical interventions with sequential therapy using 2 bypassing agents.
There were 2 deaths (D3 and P1) in our study (5.6%, 2/36); 1 patient (D3) died of intracranial hemorrhage and the other (P1) presented with an uncontrolled traumatic hemothorax. In the latter case, we performed femoral angiography followed by embolization to identify the sites of bleeding. Although the bleeding was almost controlled, the patient died of excessive retroperitoneal bleeding due to a vascular injury at the femoral site.
The 36 surgeries included 5 emergency and 31 elective surgeries (Table 5). Two patients (D3 and P1) (2/5, 40%) died after emergency surgeries. Of the remaining 3 patients (D2, M1, and R2), 2 (D2 and R2) had small bowel ischemia and the other (M1) underwent an emergency thoracotomy for hemothorax with hemostatic covering using bypassing agents. In the last 3 cases (D2, M1, and R2), postoperative bleeding control was successful after sequential therapy.
Go to : 
Neutralizing antibodies to FVIII or FIX pose problems in the management of patients with hemophilia A or B. It has been reported that both APCC and rFVIIa are effective for bleeding control in hemophilia patients with inhibitors. The hemostatic efficacy of bypassing agents has been documented; APCCs and rFVIIa have been reported to be effective in 91.2% and 84.0% of patients undergoing surgical interventions [16]. In patients with high-responding inhibitors, however, some bleeding episodes do not respond well to the initial agents because of the unreliability with which hemostasis can be achieved and maintained [1214]. In addition, the use of 2 bypassing agents is associated with a risk of thromboembolism [1011]. Thus, all but essential surgeries tend to be avoided in hemophilia patients with inhibitors. Moreover, the number of case reports of major surgeries and elective procedures is limited.
Our study reports the findings of a single-center experience in hemophilia patients with inhibitors who were undergoing surgery and receiving high-dose factor concentrates or bypassing agents. Although unexpected complications led to additional surgeries and further hospitalizations in some cases, all but 2 patients were discharged (34/36, 94.4%). There were 6 patients whose bleeding could not be well controlled despite the use of single bypassing agents. However, all of these 6 patients were successfully discharged after receiving sequential bypassing therapy. Similar reports have used the rate of patient discharge as a main outcome measure of successful surgery [1516]. Nevertheless, other measures might be useful for assessing the outcome of surgery in hemophilia patients with inhibitors, such as the patient's systemic condition, length of hospital stay, and amount of blood transfusion.
In this study, the death rate was 5.6% (2/36). However, considering emergency conditions only, the death rate was 40% (2/5). Although the same protocol was applied to all surgeries, the patient's general condition and various patient-specific factors might also have affected the general coagulation process and led to poor outcomes.
In patients with hemophilia, it is important to identify preoperative factors that may affect perioperative complications. In a report of hemophilia patients without inhibitors, the following factors could be used to identify patients potentially at risk: abnormal alkaline phosphatase, albumin, platelet, and α-fetoprotein levels; the presence of ascites; and Child-Pugh classification C [17]. That is, hemophilia patients with these perioperative risk factors might be vulnerable to higher mortality. This leads to the suggestion that laboratory measurements might be helpful for predicting postoperative outcomes in hemophilia patients with inhibitors.
One of the major challenges in managing hemophilia patients with inhibitors is predicting the product to which a patient will show a good response. Some assays, such as thrombin generation assay, thromboelastography, and clotting waveform analysis are useful for assessing the hemostatic status of hemophilia patients with inhibitors during the perioperative period. These assays have yet to be validated and have advantages and disadvantages [181920]. Despite the favorable discharge rate in this study, the initial clotting factor concentrates needed to be changed in 27.8% of the cases (10/36) (Table 4). It is important to predict which clotting factor concentrates are best for the patient and to decide whether to change the clotting factor concentrates during the postoperative period; these assays might be helpful.
For patients who have poor responses to both rFVIIa and APCC as single agents, sequential therapy using both agents was performed, but this must be used with caution because of the increased risk of thrombosis. It is thus essential to monitor conventional laboratory indicators of consumption coagulopathy, such as platelet counts, fibrinogen, activated partial thromboplastin time, prothrombin time, and D-dimers, in patients receiving a high dose of bypassing agents [514]. In some patients in our series, tests for consumption coagulopathy were checked, and the results were normal without thrombotic complications.
The management recommendations developed by the consensus group stress several considerations aimed at optimizing the safety and efficacy of bypassing agents for surgical procedures in hemophilia patients with inhibitors. Following these consensus protocols [2122], the key to the success of bypassing agents as hemostatic cover in elective surgery is to prepare patients with thorough preoperative planning. Therefore, the following points should be carefully considered before surgery: timing of the surgery, the previous clinical response to treatment, and underlying conditions of patients determined by laboratory tests such as current inhibitor titer and coagulation factors. Also, patients should be monitored closely to access hemostatic efficacy and thrombotic complications at an early stage. Above all, it is most important that the timing schedule of the administration of bypassing agents be strictly adhered to as the omission of a dose may result in bleeding.
This study investigated the total amount of clotting factor concentrates given and the period of infusion. Because the series involved different types of surgeries and various patient conditions, it was difficult to evaluate the results. Most patients were managed at several other clinics after discharge, including hemostatic and rehabilitative care. Therefore, our ability to evaluate a patient's condition after discharge was limited.
In conclusion, our results indicate that postoperative outcomes can be successful in hemophilia patients with inhibitors if bleeding is managed effectively using bypassing agents during the perioperative period. In addition, our results indicate that more active and aggressive management should be performed for patients undergoing emergency surgeries. It is thus imperative that outcome measures for successful postoperative outcomes based on the hemostatic efficacy of bypassing agents are customized for individual hemophilia patients.
Go to : 
Notes
Authors' Disclosures of Potential Conflicts of Interest: No potential conflicts of interest relevant to this article were reported.
Go to : 
References
1. Valentino LA, Cooper DL, Goldstein B. Surgical experience with rFVIIa (NovoSeven) in congenital haemophilia A and B patients with inhibitors to factors VIII or IX. Haemophilia. 2011; 17:579–589. PMID: 21294815.

2. Polyanskaya T, Zorenko V, Karpov E, Sampiev M, Mishin G, Vasiliev D. Experience of recombinant activated factor VII usage during surgery in patients with haemophilia with inhibitors. Haemophilia. 2012; 18:997–1002. PMID: 22672066.

3. Morfini M, Haya S, Tagariello G, et al. European study on orthopaedic status of haemophilia patients with inhibitors. Haemophilia. 2007; 13:606–612. PMID: 17880451.

4. Yu Polyanskaya T, Khametova RN, Yu Andreev N. Development and clinical course of hemophilia complicated by the presence of an inhibitor. Haematol Transfusiol. 2002; 47:9–12.
5. Teitel JM, Carcao M, Lillicrap D, et al. Orthopaedic surgery in haemophilia patients with inhibitors: a practical guide to haemostatic, surgical and rehabilitative care. Haemophilia. 2009; 15:227–239. PMID: 18752535.

6. Négrier C, Lienhart A, Numerof R, et al. SURgical interventions with FEIBA (SURF): international registry of surgery in haemophilia patients with inhibitory antibodies. Haemophilia. 2013; 19:e143–e150. PMID: 23282031.

7. Ingerslev J. Efficacy and safety of recombinant factor VIIa in the prophylaxis of bleeding in various surgical procedures in hemophilic patients with factor VIII and factor IX inhibitors. Semin Thromb Hemost. 2000; 26:425–432. PMID: 11092219.

8. Lauroua P, Ferrer AM, Guérin V. Successful major and minor surgery using factor VIII inhibitor bypassing activity in patients with haemophilia A and inhibitors. Haemophilia. 2009; 15:1300–1307. PMID: 19659794.

9. Witmer C, Young G. Factor VIII inhibitors in hemophilia A: rationale and latest evidence. Ther Adv Hematol. 2013; 4:59–72. PMID: 23610614.

10. FEIBA. VH Anti-Inhibitor Coagulant Complex [package insert]. Westlake Village, CA: Baxter Healthcare Corporation;2005. Accesed May 4, 2015. at http://www.baxter.com.pr/healthcare_professionals/products/feiba_vh.html.
11. Novo Nordisk A/S. NovoSeven [package insert]. Novo Nordisk A/S. Bagsvaerd, Denmark: Novo Nordisk A/S;2005. Accesed May 4, 2015. at http://www.novo-pi.com/novosevenrt.pdf.
12. Teitel J, Berntorp E, Collins P, et al. A systematic approach to controlling problem bleeds in patients with severe congenital haemophilia A and high-titre inhibitors. Haemophilia. 2007; 13:256–263. PMID: 17498074.

13. Scharrer I. Recombinant factor VIIa for patients with inhibitors to factor VIII or IX or factor VII deficiency. Haemophilia. 1999; 5:253–259. PMID: 10469179.

14. Han MH, Park YS. Sequential therapy with activated prothrombin complex concentrates and recombinant activated factor VII to treat unresponsive bleeding in patients with hemophilia and inhibitors: a single center experience. Blood Res. 2013; 48:282–286. PMID: 24466553.

15. Caviglia H, Candela M, Galatro G, Neme D, Moretti N, Bianco RP. Elective orthopaedic surgery for haemophilia patients with inhibitors: single centre experience of 40 procedures and review of the literature. Haemophilia. 2011; 17:910–919. PMID: 21342367.

16. Ludlam CA, Smith MP, Morfini M, Gringeri A, Santagostino E, Savidge GF. A prospective study of recombinant activated factor VII administered by continuous infusion to inhibitor patients undergoing elective major orthopaedic surgery: a pharmacokinetic and efficacy evaluation. Br J Haematol. 2003; 120:808–813. PMID: 12614214.

17. Hirose J, Takedani H, Koibuchi T. The risk of elective orthopaedic surgery for haemophilia patients: Japanese single-centre experience. Haemophilia. 2013; 19:951–955. PMID: 23746133.

18. Berntorp E, Collins P, D'Oiron R. . Identifying non-responsive bleeding episodes in patients with haemophilia and inhibitors: a consensus definition. Haemophilia. 2011; 17:e202–e210. PMID: 20825500.

19. Young G, Blain R, Nakagawa P, Nugent DJ. Individualization of bypassing agent treatment for haemophilic patients with inhibitors utilizing thromboelastography. Haemophilia. 2006; 12:598–604. PMID: 17083509.

20. Váradi K, Negrier C, Berntorp E, et al. Monitoring the bioavailability of FEIBA with a thrombin generation assay. J Thromb Haemost. 2003; 1:2374–2380. PMID: 14629472.

21. Giangrande PL, Wilde JT, Madan B, et al. Consensus protocol for the use of recombinant activated factor VII [eptacog alfa (activated); NovoSeven] in elective orthopaedic surgery in haemophilic patients with inhibitors. Haemophilia. 2009; 15:501–508. PMID: 19187194.

22. Rangarajan S, Austin S, Goddard NJ, et al. Consensus recommendations for the use of FEIBA(®) in haemophilia A patients with inhibitors undergoing elective orthopaedic and nonorthopaedic surgery. Haemophilia. 2013; 19:294–303. PMID: 22989234.
Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download