INTRODUCTION
Renal impairment (RI) is a common manifestation of multiple myeloma (MM). Although the incidence of RI at diagnosis varies with the definition used for renal insufficiency, this manifestation has been generally reported in ~20-40% of MM cases [
12]. In several studies, up to 10% of MM patients have severe renal failure requiring hemodialysis [
3]. In addition, RI can also evolve over time, and an estimated 25-50% of patients are affected during the course of MM [
4]. The presence of renal dysfunction has been reported to be an adverse risk factor in MM in the era of conventional chemotherapy. Patients with RI have often presented with advanced disease and high tumor burden at diagnosis. Renal dysfunction is also associated with a high risk of treatment-related toxicity and an increased risk of early death [
567]. Recently, the availability of novel agents has resulted in significant improvement of renal function in MM patients with RI [
8], and improved survival of patients with severe RI [
9]. However, some patients who present RI show poor prognosis, and few studies have examined prognostic factors for survival in RI patients treated with novel agents.
The urine flow rate is considered to be a sensitive and specific biomarker that provides an early warning signal for impending renal dysfunction, and is a predictor of mortality risk in critically ill patients [
101112]. Oliguria occurs frequently among intensive care unit patients, and is an indicator of injury to the renal parenchyma. In addition, urine flow has been proposed as a diagnostic and staging criterion for acute kidney injury. However, few studies have evaluated urine output as a prognostic factor in patients with MM presenting with RI.
In the current study, we examined the incidence of oliguria at diagnosis, and investigated the prognostic impact of oliguria for survival outcomes, in patients with MM and RI who received initial treatment with novel agents.
Go to :

MATERIALS AND METHODS
This retrospective study analyzed the records of 310 patients newly diagnosed with MM between January 2004 and November 2013 at Chonnam National University Hwasun Hospital. Among the 116 patients with RI at initial diagnosis, 14 patients who were not treated originally with novel agents were excluded. We also excluded 4 patients with active infection at initial diagnosis. Therefore, of the 310 initial MM patients, we included 98 who had RI. Renal function was assessed using the estimated glomerular filtration rate (eGFR), which was calculated by the simplified Modification of Diet in Renal Disease (MDRD) formula [
13]. RI was defined as an eGFR <60 mL/min/1.73 m
2 at initial diagnosis. Severe RI was defined as eGFR <30 mL/min/1.73 m
2. Oliguria was defined as a urine output of <0.5 mL/kg/hours for 24 hours at initial diagnosis [
14].
The degree of restoration of renal function was evaluated using recently proposed criteria [
15]. Renal complete response (CR) was defined as a sustained increase in baseline eGFR of >60 mL/min/1.73 m
2. Renal partial response (PR) was defined as an increase of eGFR from <15 to 30-59 mL/min, and renal minor response (MR) as sustained improvement of baseline eGFR from <15 mL/min to 15-29 mL/min or, if baseline eGFR was 15-29 mL/min, improvement to 30-59 mL/min. Clinical staging was performed using the International Staging System (ISS). Treatment response was assessed on the first day of each treatment cycle according to the International Myeloma Working Group criteria. This study was approved by the Institutional Review Board of Chonnam National University Hwasun Hospital.
Statistical analysis
Patient characteristics were compared using Pearson's chi-square test for discrete variables or the Mann-Whitney U-test for continuous variables. Kaplan-Meier analysis was employed to define factors affecting overall survival (OS) and progression free survival (PFS). OS was calculated from the date of diagnosis until the date of the last follow-up, or death from any cause. PFS was calculated from the start of first-line treatment to disease progression, or death from any cause. Factors included in univariate analyses were: age; gender; performance status (PS); ISS; oliguria; absolute lymphocyte count (ALC); platelet count; lactate dehydrogenase level (LDH); eGFR; renal CR; performance of autologous stem cell transplantation (ASCT); and treatment with bortezomib. Factors associated with P values <0.5 were included in multivariate regression analyses using Cox's proportional hazards model, to derive adjusted hazard ratios (HRs) of factors prognostic of OS. All statistical computations were performed using SPSS version 18.0 (SPSS, Chicago, IL). A P value <0.05 was considered to indicate statistical significance.
Go to :

RESULTS
Patients
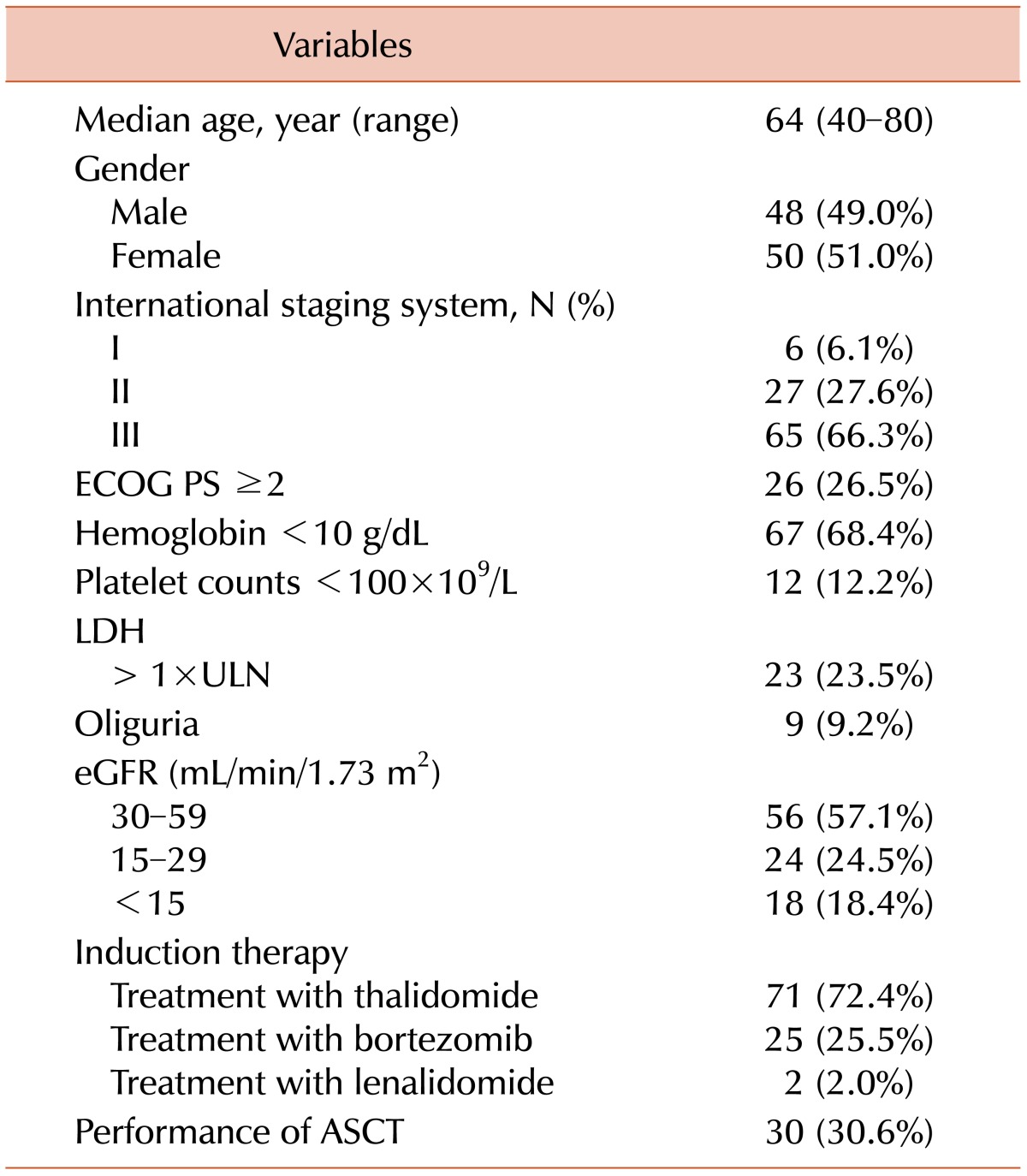
The baseline characteristics of the 98 patients are summarized in
Table 1. The median age was 64 years (range, 40-80), and 41 patients (41.8%) were >65 years old. A total of 26 patients (26.5%) had MM of the IgA type and 28.6% had light chain deposition disease. Nine patients (9.2%) presented with oliguria at initial diagnosis. Among the patients with oliguria, 3 presented with nephrotic range proteinuria (range, 4.66-7.22 g/day). One of these 3 underwent renal biopsy, and the histologic finding was consistent with myeloma cast nephropathy. Patients who did not undergo a renal biopsy showed positive results upon Bence-Jones protein testing and urine electrophoresis. The eGFR of patients with oliguria varied from 5.4-54.8 mL/min. Four patients with oliguria (44.4%) initially required dialysis. Three patients with oliguria had thrombocytopenia (<100×10
9/L) at initial diagnosis. The median eGFR was 39.7 mL/min (range, 5.1-59.8), and 43 patients (43.4%) had severe RI at diagnosis.
Table 1
Baseline characteristics (N=98).

Overall, 70 patients (71.4%) received a thalidomide-based regimen as first-line chemotherapy. Regimens included cyclophosphamide, dexamethasone, and thalidomide (CTD) in 87% of cases; melphalan, dexamethasone, and thalidomide (MPT) in 10%; and thalidomide and dexamethasone (TD) in 3%. Twenty-six patients (26.5%) received a bortezomib-based regimen as first-line therapy. These regimens included bortezomib, melphalan, and prednisolone (VMP) in 81% of cases, and bortezomib, cyclophosphamide, and dexamethasone (VCD) in 19%. Two patients (2.0%) were treated with lenalidomide and dexamethasone. Thirty patients (30.6%) underwent ASCT. One patient presenting with oliguria underwent ASCT, and showed acceptable toxicity during high-dose chemotherapy.
Treatment results and survival outcomes
The overall response rates including CR, very good partial response, and partial response, was 72.2% in patients treated with thalidomide-based regimens, 80% for those receiving bortezomib-based regimens, and 50% of those on a lenalidomide-based regimen. Improvement in renal function was observed in 84.7% of patients treated with thalidomide, and 72% of patients treated with bortezomib. Renal CR was identified in 39.8% of all patients, in 43% of patients treated with thalidomide, and in 28% of patients treated with bortezomib. Two patients treated with lenalidomide had renal CR. Among the patients with oliguria, 1 had renal CR, and 5 recovered from oliguria after front-line chemotherapy. Renal PR was observed in 29.7% of patients, and renal MR was observed in 14.3%.
Over a median follow-up period of 17.1 months (range, 1.7-100.0), the median OS was 38.7 months (95% CI 25.0-52.5) (
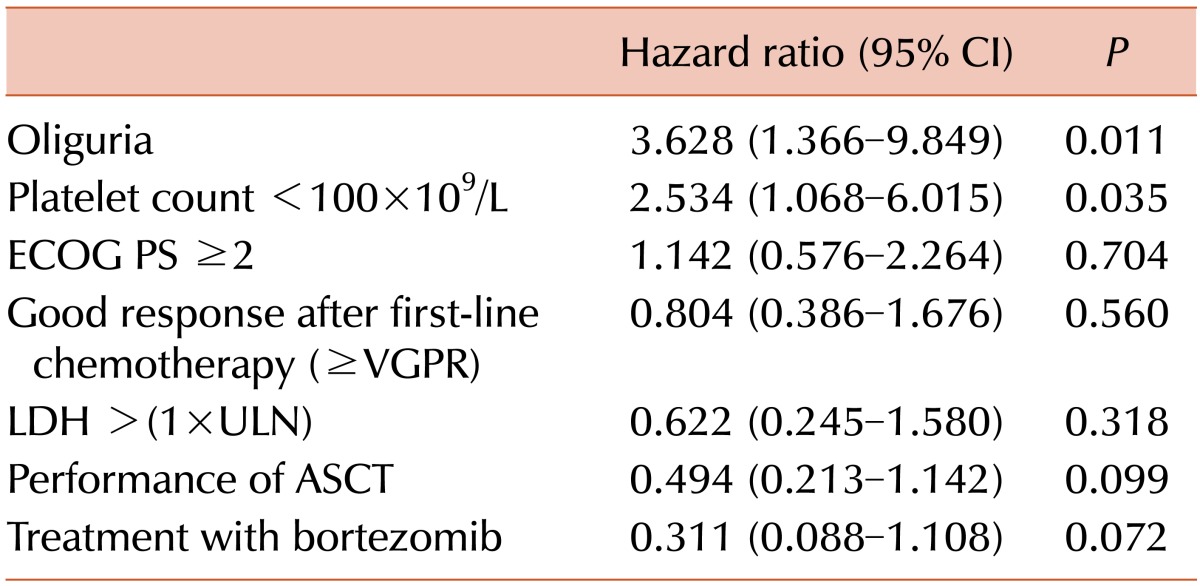
Fig. 1A). We analyzed the following variables to identify factors affecting OS: gender, PS, ISS, oliguria, ALC, platelet count, LDH levels, eGFR, serum albumin, serum β2-microglobulin, CR, performance of ASCT, and treatment with bortezomib. The univariate and multivariate analyses demonstrated that oliguria (HR 3.628, 95% CI 1.366-9.849,
P=0.011) and thrombocytopenia (HR 2.534, 95% CI 1.068-6.015,
P=0.035) were significantly associated with OS (
Tables 2 and
3). Patients with oliguria at diagnosis showed inferior survival compared to other patients (12.8 vs. 46.7 months,
P=0.010). In addition, thrombocytopenia was significantly associated with early death (9.2 vs. 47.0 months,
P<0.001) (
Fig. 1B and 1C). However, eGFR calculated by the MDRD formula was not predictive of survival outcomes in MM patients with RI (
Fig. 1D), and oliguria and thrombocytopenia did not have statistical significance on PFS (HR 1.272, 95% CI 0.558-2.895,
P=0.567, and HR 1.580, 95% CI 0.731-3.417,
P=0.245, respectively).
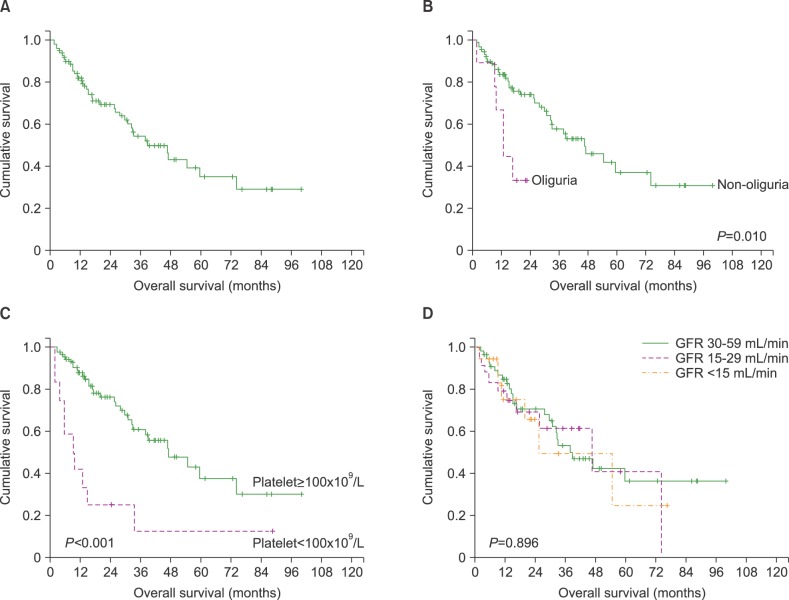 | Fig. 1Kaplan-Meier overall survival (OS) curves of all patients (A), OS according to oliguria (B) and thrombocytopenia (C), and OS per GFR criteria (D), at diagnosis.
|
Table 2
Univariate analysis of factors associated with overall survival.
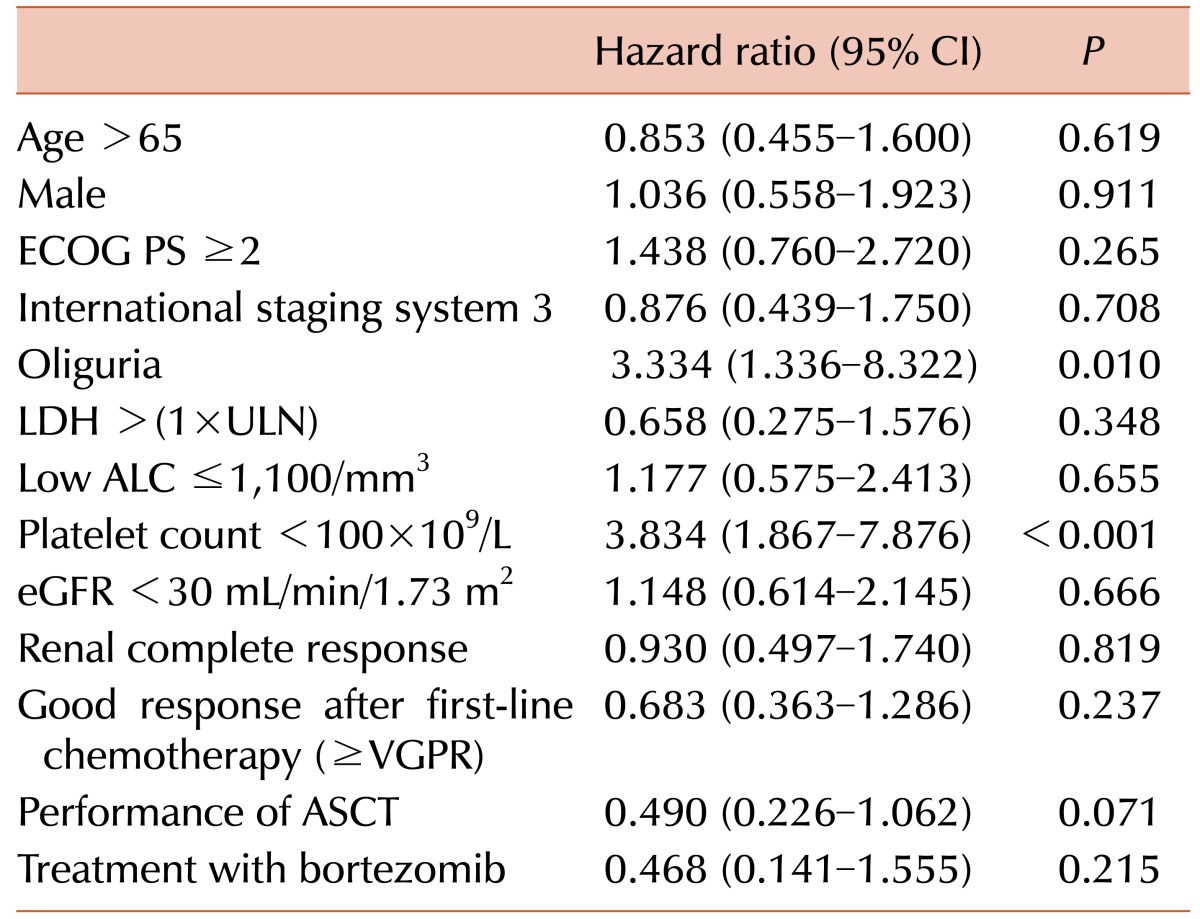

Table 3
Multivariate analysis of factors associated with overall survival.

Go to :

DISCUSSION
Serum creatinine is used to define RI, and has been utilized frequently in previous studies of MM patients to investigate the prognostic role of RI. The Durie-Salmon staging system classifies patients as having abnormal renal function when serum creatinine >2 mg/dL [
16]. However, the utility of serum creatinine as an estimate of renal function is limited because it may vary according to factors such as age, sex, and muscle mass. The eGFR calculated by the MDRD equation is a more accurate parameter, providing a true reflection of renal function [
1718]. Despite its limitations, such as a strong bias and reliance on a creatinine assay, the eGFR calculated by the MDRD formula is recommended for the evaluation of RI in patients with MM [
15]. However, it is unclear whether severe RI as identified by eGFR criteria is predictive of poor prognosis in MM. One retrospective study has shown that MM patients with eGFR <30 mL/min had poorer survival outcomes compared to those with eGFR ≥30 mL/min [
9]; however, a study of MM patients treated with novel agents reported no significant difference in early mortality for those with eGFR <30 mL/min [
8]. In the current study, eGFR calculated by the MDRD formula was not predictive of survival outcomes in MM patients with RI (
Fig. 1D). In addition, renal CR was not associated with overall survival. A previous study likewise found no association between achievement of renal CR or PR, and improved survival [
8].
A change in urine output has emerged recently as a robust biomarker of acute kidney injury and is associated with mortality in critically ill patients. An abrupt change in the level of serum creatinine is the most common indicator of acute kidney injury, and is the primary test used to evaluate eGFR. An eGFR based on serum creatinine is mainly used during steady state, and may lead to errors in estimation of kidney function for critically ill patients because adequate time is needed for creatinine to accumulate. Therefore, a change in serum creatinine is not generally considered to be a sensitive marker for the early detection of acute kidney injury. Macedo et al. [
19] found that oliguria for >12 hours, and 3 or more episodes of oliguria, were associated with an increased mortality rate in intensive care unit patients. Patients with isolated oliguria without a change in serum creatinine also had a poor prognosis. In the current study, oliguria at initial diagnosis was significantly associated with higher risk of mortality in patients with MM presenting with RI. Oliguria can develop due to dehydration, hypercalcemia, and light chain cast nephropathy, and may reflect serious renal injury and advanced disease status. Although the precise mechanisms by which oliguria may contribute to patient survival in MM are unclear, our data suggest that a change in urine output is a sensitive and specific biomarker of higher mortality in MM patients with RI.
Recently, an elevated platelet count has been reported to be a strong predictor of poor prognosis for various malignancies, including cancers of the lung, colon, rectum, and ovary [
202122]. However, a study of the relationship between platelet counts and thrombopoietic cytokines suggested that a low platelet count could be important in MM, because platelet counts were decreased in the advanced ISS stage (373×10
9/L in ISS1, 204×10
9/L in ISS2, 160×10
9/L in ISS3,
P=0.018) [
23]. Reports of the prognostic role of thrombocytopenia in MM patients with RI have been conflicting. One study found that thrombocytopenia (<130×10
9/L) was related to a poor prognosis (HR 2.150, 95% CI 1.167-3.962,
P=0.014) [
24], whereas Dimopoulos et al. [
9] reported no statistically significant difference (HR 1.52, 95% CI 0.875-2.65,
P=0.136). In our study, thrombocytopenia and oliguria at initial diagnosis were strong predictors of a poor prognosis. In addition, thrombocytopenia was significantly associated with poor outcomes even when patients without renal failure were included in the analysis (HR 3.611, 95% CI 2.124-6.138,
P<0.001). The cutoff value of thrombocytopenia used in this study was <100×10
9/L, because this threshold is generally used for patients assessed for a pathological condition [
25]. However, further research is needed to determine if thrombocytopenia has a prognostic role in the treatment of MM.
Although high dose chemotherapy followed by ASCT is an important strategy for the management of younger patients with MM, patients with renal failure are considered ineligible for ASCT because of a high risk of treatment-related toxicity. However, Parikh et al. [
26] demonstrated that performance of ASCT for patients with renal failure yielded acceptable toxicity, and resulted in improved renal function in approximately one-third of transplanted patients. In this study, performance of ASCT reduced the risk of death in patients with renal impairment (HR 0.490, 95% CI 0.226-1.062,
P=0.071), and therefore suggests that stem cell transplantation may be an important therapy for select patients with renal failure.
This study has several limitations, including a relatively small patient population drawn from a single institution. Also, 3 patients with oliguria had nephrotic range proteinuria, and further tests, including a renal biopsy, are usually required to evaluate amyloid light-chain amyloidosis (ALamyloidosis) or monoclonal immunoglobulin deposition disease (MIDD). In this study, only 1 patient underwent renal biopsy, but the possibility of AL-amyloidosis or MIDD was not ruled out in the other 2 patients. Because our analysis was retrospective, it is possible that some patients were excluded from this study unintentionally. Additionally, cytogenetic risk and serum free light chain assays were not performed. Finally, our findings should be validated in a larger patient cohort.
In conclusion, oliguria and thrombocytopenia were significantly associated with higher mortality in MM patients with RI. Therefore, close monitoring of urine output could be important in MM patients with RI, and patients with oliguria may require vigorous supportive therapy and prompt initiation of effective anti-myeloma therapy.
Go to :






 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download