INTRODUCTION
In hematopoietic stem cell transplantation (HSCT), cord blood (CB) with a high total nucleated cell (TNC) count per unit is preferred after the selection of HLA-compatible CB unit(s) for a given recipient [
1]. The TNC count is critical, because TNCs have been associated with the speed of engraftment and post-transplantation survival after HSCT. Therefore, the TNC count has been used as a selection criterion for CB units [
2,
3,
4]. Because CD34+ cells have been associated with post-transplantation survival and graft-versus-host disease, the CD34+ cell count has also been considered an important criterion for selecting CB units, in addition to the TNC count [
5,
6,
7].
CB units are cryopreserved in liquid nitrogen for several years until they are used for suitable recipients. Therefore, the quality of cryopreserved CB units should ideally be maintained during cryopreservation. Studies regarding the quality of CB units cryopreserved for a maximum of 23.5 years at CB banks in other countries have been reported [
8,
9,
10,
11,
12].
Since 1992, when the New York Blood Center's Cord Blood Program was founded [
13], many CB banks have come into existence. In Korea, 17 CB banks, including three government-assigned public CB banks, have been operating with the permission of the Ministry of Health & Welfare, according to the law on the management and research of CB proclaimed in July 2011 [
14,
15]. To date, no quality assessment of cryopreserved CB units has been conducted in domestic CB banks. Since May 2006, when the Seoul Metropolitan Government Public Cord Blood Bank (Allcord) was founded, approximately 26,000 CB units have been cryopreserved until July 2013.
In this study, we evaluated the quality of cryopreserved CB units according to the duration of cryopreservation, ranging from 1 year to 5 years, and investigated whether the quality of the cryopreserved CB units changed during cryopreservation.
Go to :

MATERIALS AND METHODS
Subject selection
Two hundred CB units unsuitable for allogeneic transplantation according to the Korean legislative guidelines were obtained from the Seoul Metropolitan Government Public Cord Blood Bank (Allcord). The 200 CB units were divided into 5 groups according to the duration of cryopreservation: 1 year, 40 units; 2 years, 41 units; 3 years, 39 units; 4 years, 41 units; and 5 years, 39 units. This study was approved by the Institutional Review Board at Boramae Hospital, after the study documents were reviewed.
CB collection, tests, and processes for cryopreservation
CB was collected
in utero in the delivery room. CB processing and efficacy testing were performed according to the standard operating procedures of the Seoul Metropolitan Government Public Cord Blood Bank (Allcord), which were described in our previous report [
16].
CB thawing and assessment of post-thaw CB parameters
After thawing the selected CB units in a 37℃ water bath, the TNC and CD34+ cell counts, aldehyde dehydrogenase (ALDH) level, cell viability, apoptosis, and the number of colony-forming units (CFUs) were examined in the thawed CB samples from each selected bag. Data on the TNC count, the CD34+ cell count, and cell viability before freezing were retrieved from the bank's database. Post-thaw testing was performed as described in our previous report [
16].
ALDH analysis
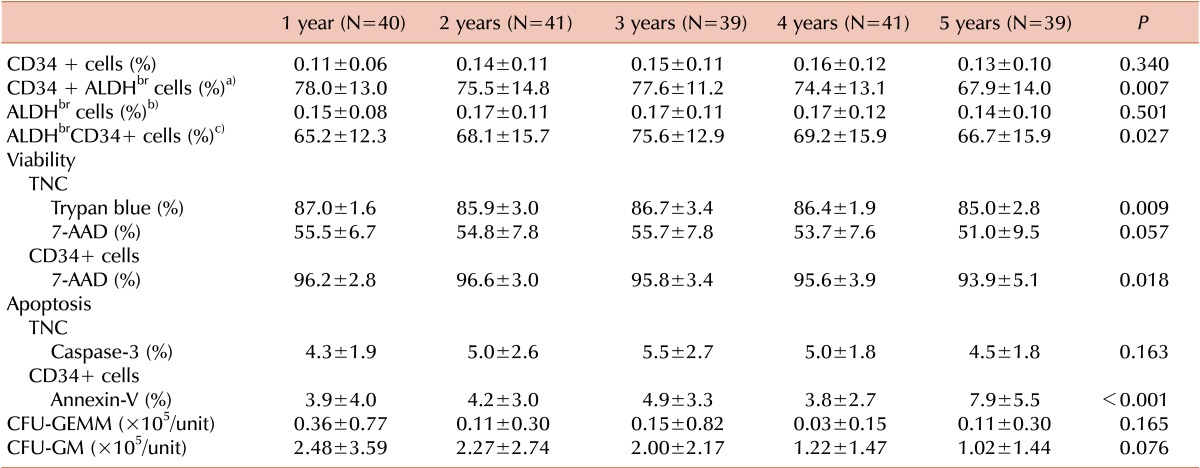
ALDH-bright cells (ALDHbr cells), which show high ALDH activity, were stained with BODIPY-aminoacetaldehyde (BAAA), the fluorescent substrate for ALDH (ALDEFLUOR; Aldagen, Durham, NC, USA). Erythrocytes were lysed using an ammonium chloride-based buffered solution. Cells were adjusted to a concentration of 1×106 cells/mL using ALDEFLUOR assay buffer (Aldagen). Aliquots of 1 mL each of the experimental cell suspension (experimental tube), and control cell suspension (control tube) were placed in labeled tubes and stained with 5 µL of BAAA at the same time. Control tube samples were additionally treated with 5 µL of diethylaminobenzaldehyde (DEAB), a specific inhibitor of ALDH. After incubation at 37℃ for 40 min, the cell suspensions were stained with 10 µL of phycoerythrin (PE)-conjugated anti-CD34 (BD Biosciences, San Diego, CA, USA) and 5 µL of phycoerythrin cyanine 7 (PC7)-conjugated anti-CD45 (BD Biosciences) and refrigerated at 4℃ for 20 min. After centrifugation at 250 g for 5 min at 4℃, the supernatant was removed, cell pellets were resuspended using 0.5 mL of ALDEFLUOR assay buffer (Aldagen), and tubes were capped and stored at 4℃ until analysis by flow cytometry (FC-500; Beckman Coulter). From each sample, 50,000 CD45+ events were acquired. The background fluorescence of the ALDHbr cells was assessed by analyzing the extent of cell inhibition by DEAB in the control tube, and ALDHbr cells were gated from the cells in the experimental tube. ALDHbr cells among CD34+ cells (CD34+ALDHbr cells) and CD34+ cells among ALDHbr cells (ALDHbrCD34+ cells) were enumerated.
Cell viability test
The viability of TNCs was measured using 0.4% trypan blue staining and 7-aminoactinomycin D (7-AAD) by excluding the non-viable cells in the process of CD34+ cell enumeration. The viability of CD34+ cells was measured using 7-AAD.
Apoptosis tests
Analysis of apoptotic cells among TNCs using caspase-3
Cells were adjusted to a concentration of 10×103 cells/µL using phosphate buffered saline (PBS). A 100 µL aliquot of the cell suspension was placed in a test tube. Cells were stained with 20 µL of fluorescein isothiocyanate (FITC)-conjugated anti-CD45 (BD, Biosciences) for 15 min at room temperature in the dark. Erythrocytes were lysed using 2 mL of an ammonium chloride-based buffered solution. After washing, the cells were adjusted to a concentration of 1×106 cells in 500 µL of the Cytofix/Cytoperm solution (BD, Biosciences) and incubated for 20 min on ice. After centrifugation, cell pellets were washed twice, using 500 µL of Perm/Wash buffer solution (BD, Biosciences), resuspended in 100 µL of Perm/Wash buffer solution, and stained with 20 µL of PE-conjugated caspase-3 for 20 min at room temperature in the dark. After washing and suspending the cells in 500 µL of Perm/Wash buffer solution, cells were analyzed by flow cytometry (FC-500; Beckman Coulter). From each sample, 50,000 events were acquired and analyzed.
Analysis of apoptotic cells among CD34+ cells using annexin-V
Cells were adjusted to a concentration of 10×103 cells/µL using PBS. A 100 µL aliquot of the cell suspension was placed in a test tube. Cells were stained with 10 µL of PE-conjugated anti-CD34 (BD, Biosciences) and 5 µL of PC7-conjugated anti-CD45 (BD, Biosciences) for 15 min at room temperature in the dark. Erythrocytes were lysed using an ammonium chloride (NH4Cl)-based buffered solution. After washing, the cells were adjusted to a concentration of 1×106 cells in 100 µL of the annexin-V binding buffer (BD, Biosciences) and stained with 10 µL of FITC-conjugated annexin-V (BD, Biosciences) for 15 min on ice in the dark. After adding 10 µL of peridinin-chlorophyll-protein complex (PerCP)-binding 7-AAD and 380 µL of annexin-V binding buffer, cells were analyzed by flow cytometry (FC-500; Beckman Coulter). From each sample, 50,000 events were acquired and analyzed from among the live cell population (negative for 7-AAD).
CFU assay
The CFU assay was performed using a commercially available methylcellulose medium (MethoCult H4435 Enriched; Stemcell Technologies, Vancouver, BC, Canada). CB samples containing 1×104 cells each were suspended in 300 µL of PBS, mixed with 3 mL of methylcellulose medium, plated on 35-mm diameter Petri dishes in duplicate, and incubated at 37℃ with 5% CO2 in a 100% humidified atmosphere for 14 days. CFU-granulocyte/macrophage (CFU-GM) and CFU-granulocyte/erythrocyte/macrophage/megakaryocyte (CFU-GEMM) were differentiated and counted according to morphology, size, and color of colonies using an inverted microscope. The mean number of colonies in the 2 dishes was calculated.
Statistical analysis
A paired t-test was performed to compare the values measured before freezing and those measured after thawing. A one-way analysis of variance (ANOVA) was performed to compare the values between the groups, and Tukey and Tamhane post-hoc tests were used for pair-wise comparisons when the ANOVA yielded statistically significant results. Differences were considered statistically significant when the P-value was <0.05. All statistical calculations were performed using the Statistical Package for Social Sciences (SPSS) v. 12.0 systems (version 12.0; IBM Corp., Chicago, IL, USA).
Go to :

DISCUSSION
In this study, which included a large number of CB units, we investigated whether the quality of CB was affected by cryopreservation.
CB is easily available for allograft transplant to suitable recipients during the HSCT procedure because CB units are cryopreserved, and hence, the quality of CB units as hematopoietic stem cell source, should be maintained during the cryopreservation period. In our previous study [
17], we analyzed the proportion of cryopreserved CB units suitable for transplantation according to recipient weight with a criterion of a minimum TNC count of more than 2.0×10
7/kg before cryopreservation [
5,
18]. The results of this previous study showed that whereas 100% of the cryopreserved CB units had a sufficient TNC count for recipients weighing less than 30 kg, only 12% of the cryopreserved CB units had a sufficient TNC counts for recipients weighing more than 60 kg [
17]. For this reason, the TNC and CD34+ cell counts in the CB units must be maintained during cryopreservation.
Some studies have reported on the quality assessment of cryopreserved CB units at CB banks in other countries, but these results differ to some extent [
8,
9,
10,
11,
12]. Broxmeyer et al. [
8,
9,
10,
11] compared TNC recovery and the numbers of CFU-GEMM and CFU-GM before and after cryopreservation and found no significant differences after CB cryopreservation for a maximum of 23.5 years. Yamamoto et al. [
12] reported that the TNC count, CD34+ cell count, and CFU-GM number decreased after cryopreservation; however, recovery of the TNC count, CD34+ cell count, and CFU-GM number did not differ significantly between CB units cryopreserved for 1 month and those cryopreserved for 10 years.
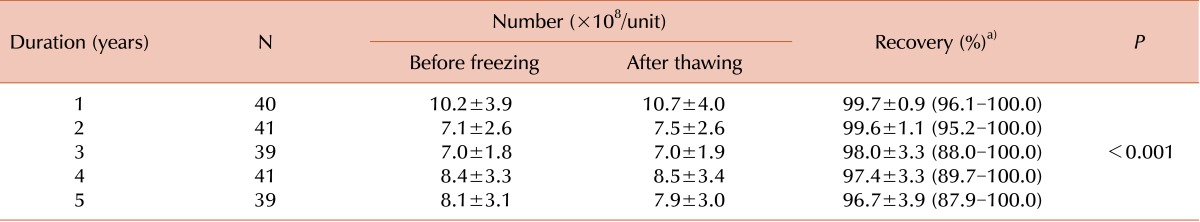
The CB units used in our study were stored for a maximum of 5 years, which is shorter than that reported in previous studies. However, while previous studies considered a small number of CB units (a maximum of approximately 20) in each group with varying durations of cryopreservation, our study was performed using a total of 200 CB units with approximately 40 units in each of the 5 groups of varying durations of cryopreservation.
TNC recovery was significantly higher in the 1-year and 2-year cryopreservation groups than in the 3-year, 4-year, and 5-year cryopreservation groups; however, the mean value of TNC recovery was more than 96.7% in all groups. Therefore, we speculated that there was no decreasing trend in TNC recovery with an increase in the duration of cryopreservation.
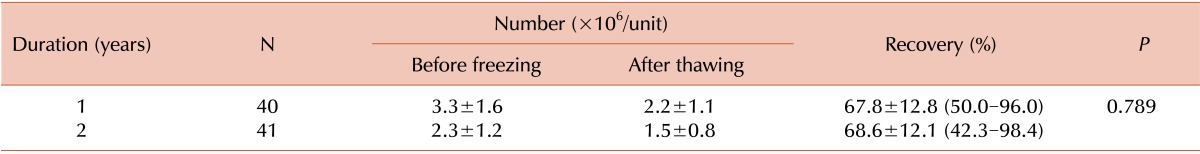
The number of CD34+ cells enumerated differs according to the flow cytometry systems and counting methods [
19]. Here, the numbers of CD34+ cells enumerated using different flow cytometry systems and different counting methods were not significantly different. However, when CD34+ cells were enumerated using the single-platform ISHAGE method, the CD34+ cell count measured using FC-500 (Beckman Coulter) was higher than that measured using FACSCalibur (BD Biosciences). Additionally, when CD34+ cells were enumerated using FACSCalibur (BD Biosciences), the CD34+ cell count measured using the single-platform ISHAGE method was higher than that measured using the dual-platform ISHAGE method. In our study, the CD34+ cell-enumeration method used after thawing was different from that used before freezing in the 3-year, 4-year, and 5-year cryopreservation groups; therefore, we could not analyze whether CD34+ cell recovery changed in these groups. However, CD34+ cell recovery in the 1-year and 2-year cryopreservation groups was approximately 68%, in agreement with the findings of previous studies on CD34+ cell recovery in cryopreserved CB and peripheral blood stem cells (PBSC) after thawing, although the duration of cryopreservation differed to some extent [
12,
20]. The mean value of CD34+ cell recovery was 72.7% in CB units cryopreserved for 1 month and 68.4% in CB units cryopreserved for 10 years [
12]. CD34+ cell recovery in PBSC products cryopreserved for a median of 142 days was 72.7% [
20]. Currently, most CB banks have a criterion for the minimal TNC count suitable for cryopreservation. However, after the large number of CB units required to cover the diversity of HLA polymorphisms is secured, a criterion for the minimal number of CD34+ cells suitable for cryopreservation would be needed. If CD34+ cell recovery after cryopreservation, which was approximately 68% in our study, is considered when defining the minimal number of CD34+ cells suitable for cryopreservation, we would be able to store high-quality CB units for HSCT.
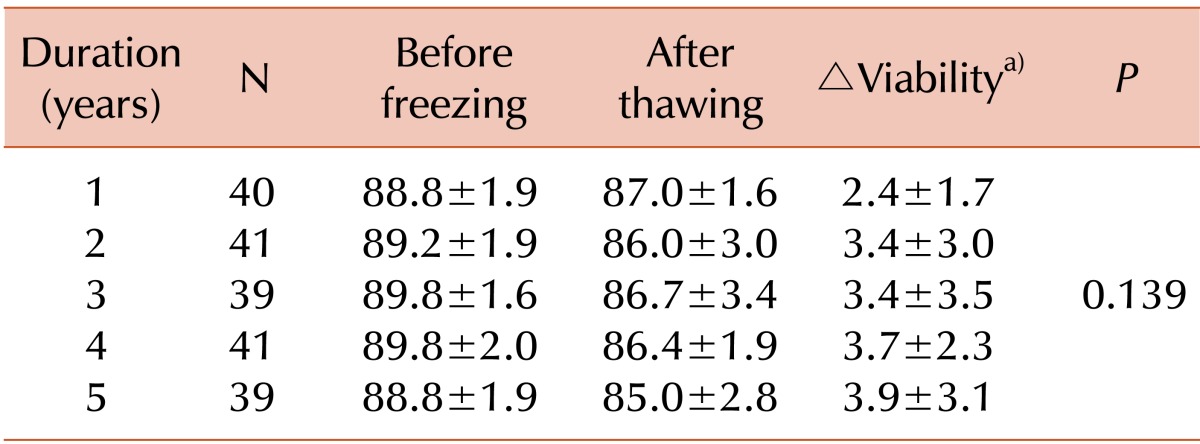
In this study, we introduced a cell-viability test using 7-AAD after thawing the CB units and found that the viability of TNC measured using 7-AAD was lower than that measured using trypan blue staining. Whereas the mean value of viability based on trypan blue staining exceeded 85.0% in all groups, the mean value of viability based on 7-AAD staining was 51.0%-55.7%. A previous study also described a discrepancy in values of TNC viability between trypan blue and 7-AAD and suggested that cell viability evaluations using 7-AAD are preferred [
21]. The viability of CD34+ cells measured using 7-AAD exceeded 93.9% in all groups; therefore, we confirm that most CD34+ cells survive cryopreservation.
As new aspects, ALDH levels and apoptosis were examined in our study as parameters for assessing the cryopreserved CB unit quality. ALDH is highly expressed in primitive hematopoietic cells, and surface markers reflecting hematopoietic stem cell activity, such as CD34 and CD133, have been reported to be expressed on some fraction of ALDH
br cells [
22]. According to previous studies, CD34+ALDH
br cells and ALDH
brCD34+ cells affect long-term xenograft and multi-lineage hematopoiesis [
22,
23,
24]. Apoptotic CD34+ cells have been reported to be able to influence transplantation outcome [
25]. Because annexin-V is attached to phosphatidylserine, which is translocated from the inner cell membrane to the outer cell membrane during early apoptosis, annexin-V is a marker of apoptotic cells. However, trypan blue and 7-AAD stain only dead cells because they only penetrate non-intact cell membranes [
26,
27]. Caspase-3 activity is increased during apoptosis [
28].
In the 5-year cryopreservation group of our study, TNC recovery, the change in cell viability, and the percentage of CD34+ALDH
br cells were low, and the viability of TNC based on trypan blue staining, the viability of CD34+ cells based on 7-AAD staining, and the percentage of apoptotic CD34+ cells were high, compared to the values in the other groups. Therefore, the quality of CB units cryopreserved for 5 years could be been decreased. However, the mean value of TNC recovery was more than 96.7% in all groups, and the mean viability of CD34+ cells was more than 93.9% in all groups. In particular, the numbers of CFU-GEMM and CFU-GM, representing the clonogenic and proliferative potentials of hematopoietic stem cells [
29,
30], did not significantly differ according to the duration of cryopreservation. Therefore, we suggest that there are no significant differences in the quality of CB units cryopreserved for up to five years, from a clinical perspective. Studies on the quality of CB units according to the duration of cryopreservation will be continued in our laboratory. In addition, future studies will be required to ascertain whether hematopoietic recovery after transplantation would be affected by the duration of cryopreservation of transplanted CB unit(s).
In summary, this study showed no significant changes in the quality of CB units over a maximum of 5 years of cryopreservation. This is the first study investigating the quality of CB units cryopreserved at CB banks in Korea. Through the results of our study, we expect that the reliability of cryopreserved CB units as a source of hematopoietic stem cells for HSCT will be improved.
Go to :





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download