TO THE EDITOR: Tumor flare reaction (TFR) is a syndrome that clinically presents with painful lymph nodes and/or spleen enlargement, and it can be accompanied by fever, rash, and clear lymphocytosis [1]. Medical literature frequently points out its association with chronic lymphocytic leukemia (CLL) and lenalidomide treatment. In a study by Chanan-Khan et al. [2], it was observed that of 26 (58% of 45 patients) CLL patients who were administered 25 mg/day oral lenalidomide treatment for 21 days in 28-day cycles developed TFR reactions (50% developed grade 1-2 reactions, and 8% developed grade 3-4 reactions). In another study by Ferrajoli et al. [3], it was observed that 10 (28% of 35 patients) CLL patients who were first treated with a dose of 10 mg/day of lenalidomide and then with an increased dose of 25 mg/day developed TFR. While TFR cases after lenalidomide treatment have been frequently reported, TFR has not been reported after Hyper-CVAD plus Rituximab (R-HCVAD) treatment. Herein, we report a patient diagnosed with mantle cell lymphoma who developed TFR after use of high dose cyclophosphamide, doxorubicin, adriamycin and dexamethasone plus rituximab.
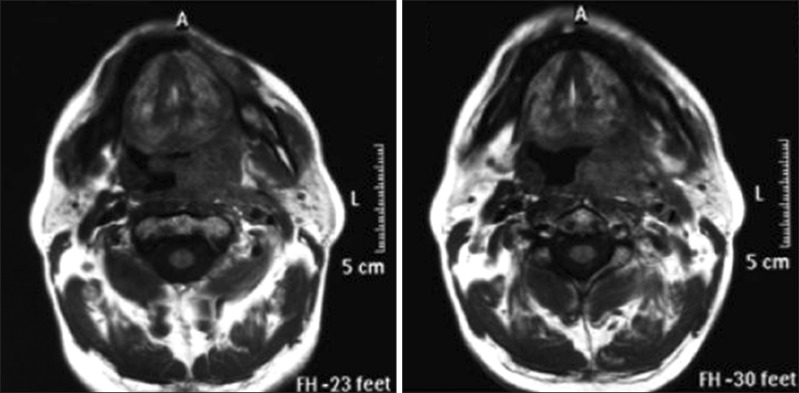
The patient, who was being followed up for relapse of mantle cell lymphoma, developed fever, throat swelling, pain, and difficulty in swallowing after R-HCVAD treatment. Magnetic resonance imaging (MRI) of the patient's throat showed a mass, sized approximately 65×29 mm at its broadest point, with its borders not clearly identified; the mass caused distinct asymmetry in the hypopharynx and oropharynx, displaced the uvula to the right side, and continued to the epiglottis level. Distinct hypertrophy of the lingual and left palatine tonsils as well as of multiple lymph nodes on both sides were observed in the left submandibular area of the cervical chain of the neck, the biggest of which was sized 20×12.5 mm (Fig. 1). These findings suggested TFR after chemotherapy, and dexamethasone (24 mg/day) was initiated. The patient's fever decreased, and his complaints regressed on the 10th day of the treatment; throat MRI revealed edema and inflammation regression when compared to that observed on the previous MRI scan. A thin area of loculation was observed in the left retropharyngeal and parapharyngeal space and a small mass residue, 2.5×8 mm in size, was observed in the parapharyngeal space, with no clear contrast involvement. The patient's complaints abated completely after dexamethasone treatment; therefore, medications were stopped and follow-up was initiated.
Until recently, it was thought that TFR associated with lenalidomide was a condition specific only in CLL patients. However, in 2010, Corazzelli et al. [4], reported three patients with relapsing or refractory Hodgkin lymphoma who developed TFR, and thus, showed that it was not so. Similarly, Eve and Rule [5], showed that three of 25 patients with mantle cell lymphoma who were treated with lenalidomide developed TFR. It was observed that two of the mantle cell lymphoma patients who developed TFR had a mild condition and recovered without any treatment. However, the other patient died of cerebrovascular complications although he was hospitalized owing to the severity of his condition (he had a prolonged reaction and his pain could not be controlled) [5]. While the etiology of TFR is not clearly known, it is thought that some mechanisms may be because of secondary production of cytokines. Studies have shown that lenalidomide does not directly induce apoptosis of or cytokine production by tumor cells, but depending on the dose, it does increase CD40, CD80, CD86, HLA-DR, and CD95 expression by activating B cells [1]. It has been shown that there is distinctive CD40 and CD86 expression in CLL cells on ex vivo lenalidomide administration, and that the expression is associated with TFR [1]. Andritsos et al. [1] have shown that, on pre- and post-treatment evaluation of the lymph nodes, infiltration of Ki67- and CD3-positive, CD8-positive, granzyme B-positive T cells increases. It has been observed that, in patients with Hodgkin lymphoma who are treated with lenalidomide and who develop TFR, cytokines (particularly interleukin [IL]-6, IL7, APRIL, BAFF/BLyS) are released because of B cell activation, and levels of free light chains increase distinctively; these changes are reflected in the clinical properties. Likewise, it has been shown that when the TFR subsides, cytokines and light chain levels decrease to normal levels [5]. Therefore, for patients who develop TFR, it is suggested that dexamethasone be used, as it provides distinctive improvement by especially inhibiting CD40 regulation [1]. The mechanism through which the R-HCVAD treatment leads to TFR is not known, but one of the above-listed mechanisms may play a role.
References
1. Andritsos LA, Johnson AJ, Lozanski G, et al. Higher doses of lenalidomide are associated with unacceptable toxicity including life-threatening tumor flare in patients with chronic lymphocytic leukemia. J Clin Oncol. 2008; 26:2519–2525. PMID: 18427150.

2. Chanan-Khan A, Miller KC, Musial L, et al. Clinical efficacy of lenalidomide in patients with relapsed or refractory chronic lymphocytic leukemia: results of a phase II study. J Clin Oncol. 2006; 24:5343–5349. PMID: 17088571.

3. Ferrajoli A, Lee BN, Schlette EJ, et al. Lenalidomide induces complete and partial remissions in patients with relapsed and refractory chronic lymphocytic leukemia. Blood. 2008; 111:5291–5297. PMID: 18334676.

4. Corazzelli G, De Filippi R, Capobianco G, et al. Tumor flare reactions and response to lenalidomide in patients with refractory classic Hodgkin lymphoma. Am J Hematol. 2010; 85:87–90. PMID: 20029955.

5. Eve HE, Rule SA. Lenalidomide-induced tumour flare reaction in mantle cell lymphoma. Br J Haematol. 2010; 151:410–412. PMID: 20880113.

Fig. 1
The patient's throat magnetic resonance imaging scan showing a heterogeneously dense mass, sized approximately 65×29 mm at its broadest point, with its borders not clearly identified; therefore, the mass caused distinct asymmetry in the hypopharynx and oropharynx, displaced the uvula to the right side, and continued to the epiglottis level.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download