TO THE EDITOR:
Plasmacytic differentiation is not a rare feature of extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma), and serum paraproteinemia is detected in more than a third of patients, suggesting that at some point, the distinction from plasma cell neoplasm (PCN) is ambiguous [1, 2]. Moreover, the endoscopic findings may be very subtle in the early stages and appear slightly nodular or atrophic, indicating the lack of a consistently clear morphologic distinction from chronic gastritis [2, 3]. A focus on a predominantly nodal presentation, serum monoclonal gammopathy, and plasmacytoid histology can lead to a wrong diagnosis such as PCN or lymphoplasmacytic lymphoma (LPL), especially when Mott cells, the characteristic hypersecretory plasma cells, are predominant in a small lymph node biopsy.
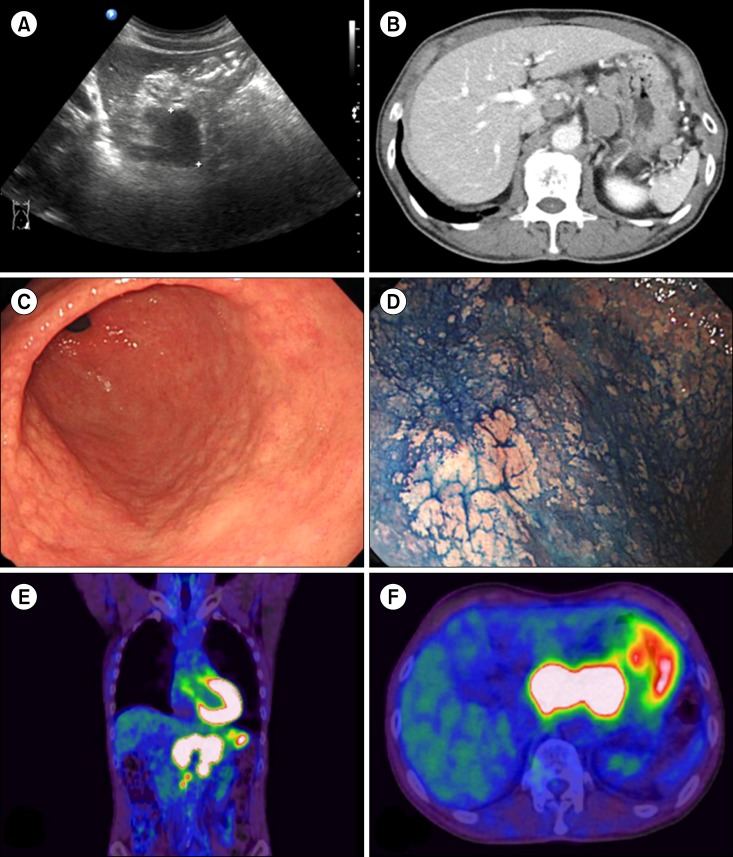
Recently, we encountered the first such case in a generally healthy elderly man who was initially diagnosed with PCN or LPL. This 72-year-old man with no specific symptoms presented with a mass-like lesion on the tail of the pancreas, incidentally detected on ultrasonography during a routine health examination (Fig. 1A). The patient's serum lactate dehydrogenase and creatine phosphokinase levels were elevated at 728 IU/L and 395 U/L, respectively, although the level of carbohydrate antigen 19-9, a pancreatic carcinoma marker, was within the normal range (16 U/mL). Subsequent computed tomography (CT) imaging revealed that the mass-like lesion was actually not a pancreatic mass but rather conglomerated, enlarged lymph nodes (4 cm in total) around the pancreas (Fig. 1B). In addition, multiple paraaortic and abdominal lymph nodes were enlarged, which suggested disseminated nodal lymphoma. A percutaneous core needle biopsy was obtained from an enlarged lymph node near the stomach.
Upon microscopic examination, a diffuse proliferation of monotonous small atypical lymphocytes and striking infiltration of hypersecretory plasma cells with eosinophilic intracytoplasmic immunoglobulin globules (Russell bodies), the so-called "Mott cells" or "grape-like cells" (Fig. 2A), were observed. As Mott cells are often considered a good diagnostic indicator of lymphoma with pathologically distinct plasmacytic differentiation, the possibility of PCN or LPL was suspected. Subsequent serum immunoglobulin quantification also revealed kappa light chain restriction (20.30 mg/L).
Gastroduodenoscopy and colonoscopy were performed concurrently to evaluate a possible primary lesion in the gastrointestinal tract. Only a few adenomatous polyps, however, were found in the colon, and a non-specific mucosal nodularity was noted in the stomach (Fig. 1C, D). Multiple random biopsies of this lesion were performed.
Surprisingly, Mott cell proliferation similar to that in the abdominal lymph node was observed in 6 of 8 biopsied samples (Fig. 2B). Upon closer observation, diffuse proliferation of monotonous small atypical lymphocytes that effaced normal glandular structures in the gastric mucosa was noted. These lymphocytes had infiltrated glandular structures to form lymphoepithelial lesions. Extensive plasma cell infiltration with striking features of intracytoplasmic immunoglobulin globules was observed.
Immunohistochemical staining of the abdominal lymph node revealed diffuse strong CD20 positivity in the atypical small lymphocytes and CD3 positivity in a few entrapped T cells (Fig. 2C). The Mott cells were strongly CD138 positive and IgG and kappa light chain positive but lambda light chain negative, indicating light chain restriction (Fig. 2D, E). Interspersed histiocytes were CD68 positive, and dendritic cells were CD23 positive. Additionally, IgG4 (used to exclude IgG4-related diseases), CD5 and cyclinD1 (to exclude mantle cell lymphoma), CD56, and S-100 were all negative. MUM-1, which is known to be associated with B-cell activation and proliferation, was strongly positive, and the Ki-67 labeling index was 35%.
Immunohistochemical staining of gastric mucosal tissues revealed a similar immunohistochemical profile. Immunohistochemical staining for pan-cytokeratin (CK AE1/AE3) highlighted lymphoepithelial lesions (Fig. 2F).
Accordingly, this case was finally diagnosed as a MALT lymphoma with predominant nodal involvement and extensive plasmacytic differentiation, including light chain restriction. Additional positron emission tomography (PET)-CT highlighted the primary lesion; this exhibited only mild to moderate uptake with slight thickening along the gastric wall and a mass-forming lesion with severe uptake encasing the aorta (Fig. 1E, F).
Fluorescence in situ hybridization was negative for t(11;18)(q21;q21), t(1;14)(p22;q32), and t(14;18)(q32;q21). Warthin-Starry staining revealed Helicobacter pylori in the gastric foveolar epithelium. Serum and urine protein electrophoresis indicated total proteins within normal ranges without remarkable fractional changes, which excluded PCN.
Nodal involvement of MALT lymphoma is relatively rare and is observed in approximately 5% of cases [2, 3, 4]. Predominant nodal involvement presenting as a mass without an obvious mucosal lesion (stage II2E) may be even rarer and can sometimes mimic other types of nodal lymphomas because these tumors may exhibit gastrointestinal dissemination in more than 25% of cases [3]. For this reason, a thorough evaluation of nodal and extranodal lesions involving various approaches, including PET-CT, endoscopy, and pathologic examination, cannot be disregarded [1].
Plasmacytic differentiation may be an important histologic clue for the differential diagnosis. PCNs (including multiple myeloma and solitary plasmacytoma), LPL, and Russell body gastritis should be included in the differential diagnosis [1, 2, 3, 5]. Notably, however, MALT lymphoma may frequently exhibit plasmacytic differentiation [1, 2, 3]. Approximately a third of MALT lymphomas are known to exhibit plasmacytic differentiation, and these mostly demonstrate monoclonal immunoglobulin production by accompanying plasma cells [2, 3, 5, 6]. Although Mott cell formation (Russell body formation) in gastrointestinal lesions has been documented quite frequently, no previous report has documented these findings in disseminated lymph nodes, which lends great interest to the present case [7, 8]. In 2013, Butrym et al. [5] reported a similar case of small B-cell lymphoma with marked plasmacytic differentiation from an inguinal lymph node biopsy. However, they failed to prove monoclonality or the presence of lymphoproliferative lesions in the gastrointestinal tract, and therefore, could only confirm the case as merely "unclassifiable" small B-cell lymphoma with Mott cell formation. In contrast, we confirmed our case to be MALT lymphoma by identifying obvious lymphoepithelial lesions and light chain restriction.
A differential diagnosis from PCN can be made by excluding the presence of paraproteinemia via serum protein electrophoresis [9, 10, 11]. Evaluations of bone marrow involvement, anemia, and any lytic bone lesions should be performed in addition to excluding other B-cell lymphomas. However, it is important to note that monoclonal gammopathy can be also observed with other lymphomas.
A differential diagnosis from LPL may be difficult if there are no specific lesions at other sites, such as the stomach [1, 2, 12]. Other than differences in preferred anatomical sites, a clear distinction between LPL and MALT lymphoma with plasmacytic differentiation is not always possible [2]. In the present case, however, although many small lymphocytes and plasma cells were observed in the lesion, there were no "plasmacytoid" lymphocytes in any of the examined tissues. In addition, although clear lymphoepithelial lesions as well as light chain restriction (especially IgG kappa) were observed, IgM, which is common in LPL, was not observed [2, 12, 13]. Histiocyte accumulation between small lymphocytes and accompanying dendritic cells in the present case also favored the diagnosis of MALT lymphoma [1, 2, 3].
Russell body gastritis, a benign inflammatory condition of the stomach characterized by extensive plasma cells with Russell bodies, has been recently described [14, 15]. Such a diagnosis from stomach biopsy findings would be important for a differential diagnosis. However, in the present case, immunohistochemically proven light-chain restriction and the presence of nodal dissemination easily excluded this unknown condition.
In summary, nodal dissemination of MALT lymphoma can mimic nodal lymphoma, especially when the primary site is grossly indistinct. Plasmacytic differentiation may often be present with MALT lymphoma; an associated extreme form such as Mott cell formation, which strongly suggests PCN or LPL, may also be observed in MALT lymphoma. An exhaustive evaluation, including PET-CT, protein electrophoresis, and immunohistochemical staining, should be conducted for an accurate diagnosis.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download