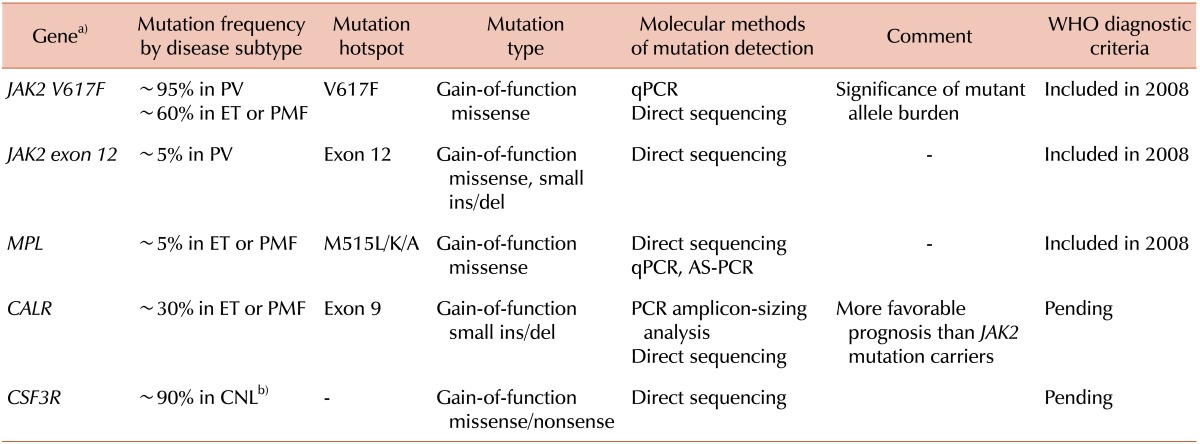
Polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (PMF) are collectively called "Philadelphia-negative classical myeloproliferative neoplasms" (MPNs), and the discovery of the JAK2V617F mutation in 2004 led us to make new progress in the diagnostic approach and therapeutic strategy for MPNs. Thereafter, other clonal markers, such as mutations of the MPL or CALR genes, were discovered and listed as useful markers for the distinction of MPN from reactive myeloproliferation.
The identification of recurrent gain-of-function somatic mutations in the
JAK2 and
MPL genes during the years 2005-2007 provided critical insights into non-
BCR/ABL1 MPNs that have advanced our understanding of the molecular pathophysiology of these diseases. The
JAK2 and
MPL mutations were readily incorporated into the diagnostic criteria for PV, ET, and PMF in the 2008 World Health Organization (WHO) classification [
1]. Thereafter, the growing application of high-throughput sequencing technologies to the identification of genetic alterations at the nucleotide level has revealed a long list of genes, other than
JAK2 and
MPL, which are mutated in MPNs. It is expected that 2 of these genes,
CALR and
CSF3R, will be added to the upcoming WHO classification, based on their significant frequency of occurrence and genotype-phenotype correlation, particularly in terms of therapeutic and prognostic implications [
2]. The
CALR gene will appear in the diagnostic criteria for ET and PMF;
CALR mutations are reported to be detected in ~70% of
JAK2-nonmutated ET and ~85% of
JAK2-nonmutated PMF.
CALR encodes the protein calreticulin, which has multiple functions and plays roles in, for example, cell proliferation and apoptosis.
CALR mutations occur exclusively in exon 9 (the last exon) and are most commonly small deletions or insertions, with or without substitutions. The two most common mutations that account for ~80% of all
CALR mutations, c.1092_1143del (p.L367fs
*46) and c.1154_1155insTTGTC (p.K385fs
*47), have been designated as type 1 and 2, respectively. The mutations both result in a frameshift to an alternative reading frame and generate a novel amino acid sequence at the C-terminus of the protein. Clinically, compared to patients with
JAK2 mutations, patients with
CALR mutations have a higher platelet count, lower Hb and leukocyte levels, a lower risk of thrombosis, and a more indolent disease course [
3]. Interestingly, mutation type was found to be associated with disease subtype (predilection for type 1 mutations in PMF) and with the clinical course of the disease (shorter survival with type 2 mutations in PMF) [
4]. Technically, PCR and amplicon-sizing analyses can detect
CALR mutations with high sensitivity and provide quantitative information, and direct sequencing analysis can then fully characterize the mutations. Of note, the 3 major driver mutations in non-
BCR/ABL1 MPNs,
JAK2,
MPL, and
CALR, occur in an almost mutually exclusive manner in PV, ET, and PMF. In addition, a mutation of the
CSF3R gene that encodes the receptor for colony stimulating factor 3, a cytokine that controls the production and differentiation of granulocytes, was shown to be an important clonal marker of chronic neutrophilic leukemia (CNL), a very rare disease entity among MPNs, based on its high frequency (~90%) in CNLs.
CSF3R mutations are also gain-of-function mutations, either missense mutations that affect the membrane-proximal extracellular domain of the protein or mutations that lead to the truncation of the protein's cytoplasmic tail. In addition to the diagnostic implications for CNL,
CSF3R mutation suggests the possibility of a therapeutic response of the mutation-bearing leukemic cells to tyrosine kinase inhibitors. Direct sequencing analysis can detect both forms of
CSF3R mutation.
Table 1 summarizes the updated knowledge of major driver mutations in MPNs.
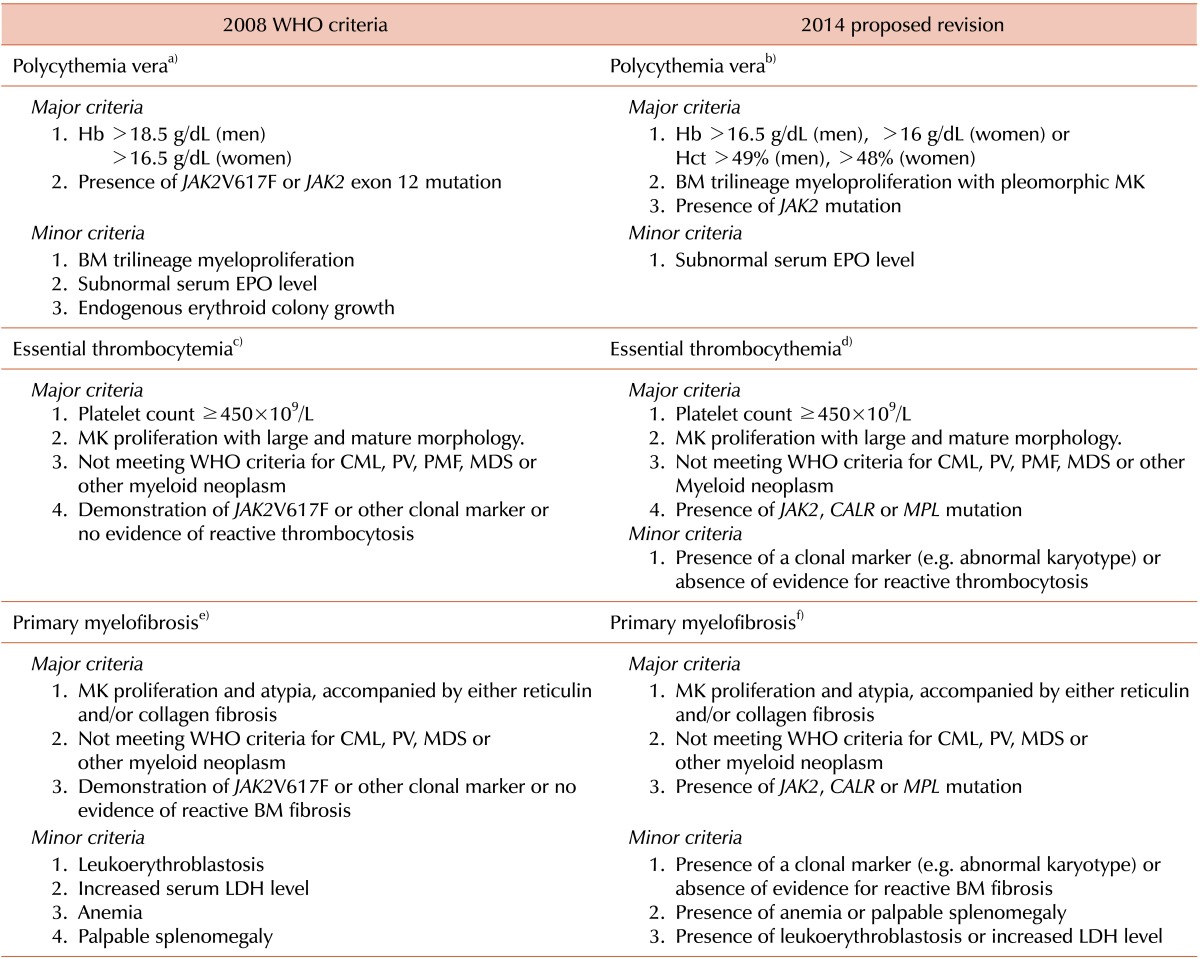
It is inevitable that the diagnostic criteria that we are using, currently, the 2008 WHO criteria, for the diagnosis of MPNs, will be refined. However, controversies and debate regarding diagnostic criteria still persist; for example, the reliability of the Hb level as a surrogate marker for increased red cell mass has been challenged in the diagnosis of PV. To resolve these challenging issues, proposals for the revision of the WHO diagnostic criteria were recently published [
2]. The most remarkable changes are proposed in the diagnostic criteria for PV (
Table 2). First, the cut-off value for the Hb level was lowered and Hct level was listed as a new criterion. Based on a study that distinguished
JAK2-mutated ET from masked PV [
5], an Hb level of 16.5 g/dL for men and 16 g/dL for women, or an Hct level of 49% in men and 48% in women, were determined to be the optimal cut-offs. Second, bone marrow morphology was promoted to a major criterion from a minor criterion, validating bone marrow morphology in PV diagnosis. Third, the endogenous erythroid colony formation test was deleted from the list of minor criteria because it is time-consuming and not generally available [
6]. Finally, for a rare case of
JAK2-nonmutated PV, a subnormal erythropoietin level was included as a minor criterion. For ET, the revised criteria include
CALR or
MPL, as well as
JAK2 mutation, and one minor criterion (presence of a clonal marker, e.g. abnormal karyotype, or absence of evidence for reactive thrombocytosis) was added for cases in which there are no
JAK2/CALR/MPL mutations (
Table 2). For PMF, a revised criterion also includes
CALR or
MPL, as well as
JAK2 mutation. In the absence of
JAK2/CALR/MPL mutations, the first minor criterion (presence of a clonal marker, e.g. abnormal karyotype, or absence of evidence for reactive BM fibrosis) aims to exclude the possibility of non-clonal bone marrow fibrosis. The second criterion (presence of anemia or palpable splenomegaly) and the third criterion (presence of leukoerythroblastosis or increased LDH level), which includes the lactose dehydrogenase level, reinforce the morphologic expression of PMF [
2] (
Table 2).
We hope that the revised WHO criteria will enable us to identify patients with MPNs early and efficiently.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download