A 55-year-old man presented with long-standing epistaxis, recent melena, and dizziness. He had a three-generation family history of chronic epistaxis, anemia, and regular blood transfusions for 16 years (
Fig. 1). Physical examinations showed mucocutaneous telangiectasias of the lips, tongue, finger tips, and lower eyelids (
Fig. 2A), along with finger clubbing and pale conjunctivae. Initial laboratory findings were 6.0 g/dL hemoglobin (90 fL MCV, 27 pg MCH), 308×10
9/L platelets, 15.4% red blood cell distribution width, 6.4% reticulocytes, 25 µg/dL serum iron, and 13.17 ng/mL serum ferritin. Endoscopic gastroduodenoscopy (EGD) and colonoscopy were performed for the anemia workup, during which numerous telangiectasia of the total gastric mucosa with old blood clots and easy touch bleeding were observed (
Fig. 2B). We treated the patient using an argon plasma coagulator (APC) (
Fig. 2B), which successfully stopped the melena. During the hospitalization period, total 8 units of red blood cell were transfused. However, he was readmitted 1 month after discharge with dizziness and anemia because of repeated gastrointestinal bleeding. Repeated endoscopic treatment with APC of multiple gastric telangiectasias was performed. Concurrently, other bleeding foci were screened using a red blood cell nuclear scan, and radioactivity was detected in the stomach, jejunum, ileum, and upper small bowel areas on both dynamic and 24-h delayed images. We needed another 8 units of red blood cell transfusion to raise hemoglobin level. As treatment methods with oral iron and endoscopic APC both failed to raise hemoglobin levels, we considered using the anti-VEGF antibody, bevacizumab. The patient first refused bevacizumab treatment due to financial problems. It was only after several failures to control local bleeding with endoscopic APC that he finally agreed to the bevacizumab treatment. Standard regimens for HHT treatment using bevacizumab have not been established, and the minimum intravenous bevacizumab dosage is >5 mg/kg in almost all reported cases. We decided to administer low-dose intravenous bevacizumab (2 mg/kg) considering his financial status. The patient received four courses of intravenous bevacizumab, 2 mg/kg per course, every 3 weeks. Despite the low bevacizumab dosage, both hemoglobin and serum ferritin levels increased and maintained higher levels after treatment, and the frequency of red blood cell transfusions decreased beginning at the early phase of treatment (
Fig. 3). He had been transfused with more than four units of packed red blood cells every 2 weeks before the bevacizumab treatment. After the low-dose intravascular bevacizumab administration, he did not need blood transfusions for 2 months. Follow-up EGD also demonstrated improvement in the numerous telangiectasias of the gastric mucosa (
Fig. 2C). However, blood transfusions resumed after the fourth course of bevacizumab treatment. Nevertheless, the frequency of blood transfusions was reduced by half (every month), and epistaxis frequency per month decreased by 50% (
Fig. 3). The patient could not continue treatment because of his financial status.
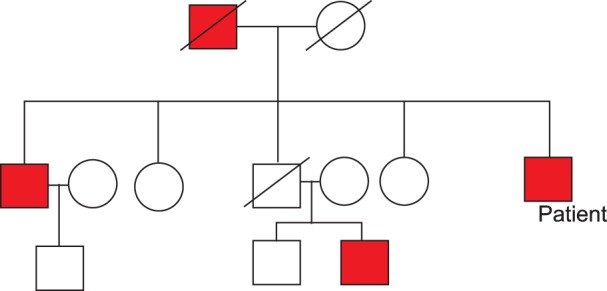 | Fig. 1Pedigree of the patient's family. Red box indicates those affected by hereditary hemorrhagic telangiectasia. 
|
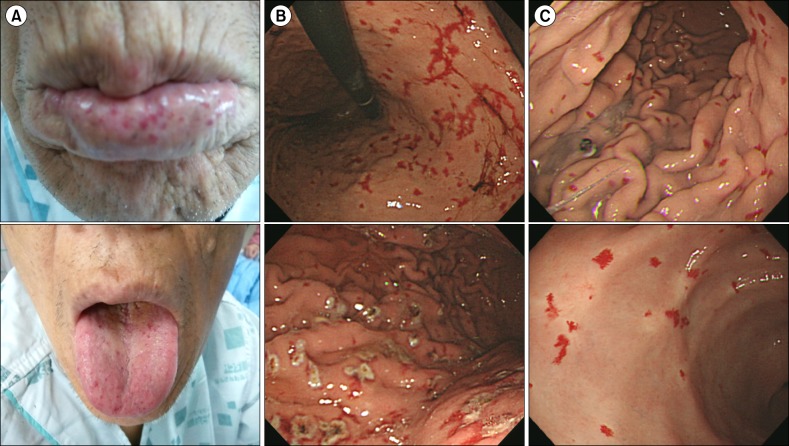 | Fig. 2(A) The lips and tongue showed mucocutaneous telangiectasias. (B) Numerous telangiectasia lesions of the total gastric mucosa with old blood clots and easy touch bleeding patterns were observed by endoscopic gastroduodenoscopy (EGD). Argon plasma coagulation (APC) provided successful management. (C) Follow-up EGD showed improved telangiectasia of the gastric mucosa after bevacizumab therapy. 
|
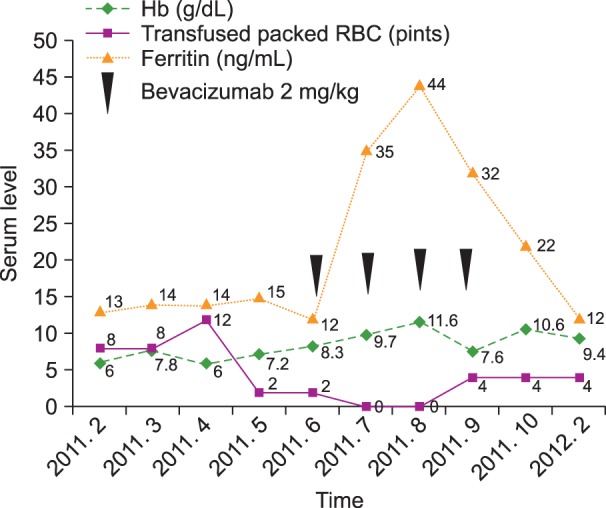 | Fig. 3Hemoglobin and serum ferritin levels increased after administration of low-dose intravenous bevacizumab and were maintained. The frequency of red blood cell transfusions decreased. However, the hemoglobin level again decreased, and blood transfusions were required after the fourth bevacizumab cycle. 
|







 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download