Abstract
Background
The number of CD34+ cells in a peripheral blood stem cell collection is the key factor in predicting successful treatment of hematologic malignancies. Korean Red Ginseng (KRG) (Panax ginseng C.A. Meyer) is the most popular medicinal herb in Korea. The objective of this study was to determine the effect of KRG on hematopoietic colony formation.
Methods
Bone marrow (BM) samples were obtained from 8 human donors after acquiring informed consent. BM mononuclear cells (MNCs) were isolated, and CD34+ cells were sorted using magnetic beads. The sorted CD34+ cells were incubated with or without total extract of KRG (50 µg/mL, 100 µg/mL) or Ginsenoside Rg1 (100 µg/mL), and the hematopoietic colony assay was performed using methylcellulose semisolid medium. The CD34+ cell counts were measured by a single platform assay using flow cytometry.
Results
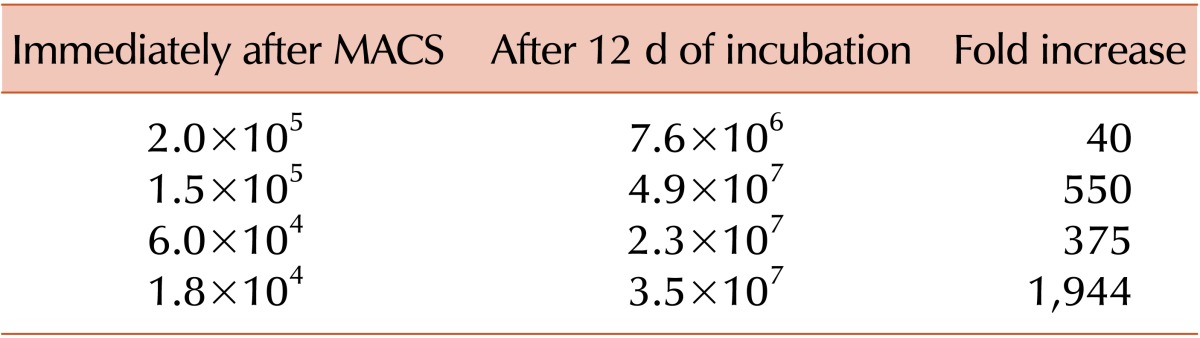
The numbers of human BM-MNCs and CD34+ cells obtained after purification were variable among donors (5.6×107 and 1.3-48×107 and 8.9×104 and 1.8-80×104, respectively). The cells expanded 1,944 times after incubation for 12 d. Total extract of KRG added to the hematopoietic stem cell (HSC)-specific medium increased CD34+ cell counts 3.6 times compared to 2.6 times when using HSC medium alone. Total numbers of hematopoietic colonies in KRG medium were more than those observed in conventional medium, especially that of erythroid colonies such as burst forming unit-erythroid.
Go to : 
Autologous or allogeneic peripheral blood stem cell transplantation (PBSCT) is the treatment of choice for hematologic malignancies such as acute myelogenous leukemia, acute lymphoblastic leukemia, malignant lymphoma, and multiple myeloma [1]. The number of CD34+ cells in the collected peripheral blood stem cells (PBSC) from patients or allogeneous donors is a key factor for predicting successful treatment [2, 3]. Chemotherapeutic agents, which are mandatory for patients with hematologic malignancies before PBSC collection, might affect CD34+ cell counts [4, 5, 6].
In Korea, Panax notoginseng saponin (PNS), derived from Panax ginseng C.A. Meyer, includes fresh ginseng, white ginseng, and red ginseng. Among them, Korean red ginseng (KRG) is one of the most popular herbal medicines in Korea. The unique components of KRG are 20(S)-ginsenoside Rg3; ginsenoside Rh2, Rs1, or Rs2; Rs3, Rs4, and Rg5 plus notoginsenosied-R4 in the protopanaxadiol group; and 20(S)-ginsenoside Rg2, 20(S)-ginsenoside-Rh1, and ginsenoside Rh4 and F4 in the protopanaxatriol group [7]. Although controversial, it is believed that PNS has antioxidant, anti-apoptotic, and anti-inflammatory properties [8].
Most studies investigating the medicinal properties of PNS have used a separate component of PNS such as Rg1, Rg2, or Rb1 to examine the anti-inflammatory or anti-apoptotic effects [9, 10, 11]. There have been a few reports about the proliferative effect of PNS on hematopoietic progenitors in traditional Chinese medicine. PNS may play a role in upregulation of gene expression related to the proliferation of hematopoietic cells by increasing the nuclear transcription factor of the glucocorticoid receptor synthesis and its DNA binding activity [12]. It has been reported that total saponin of Panax ginseng (TSPG) may promote in vitro development of erythroid-lineage cells from purified CD34+ hematopoietic stem/progenitor cells (HSC) derived from the human umbilical cord [13]. TSPG in combination with hematopoietic growth factors promotes the erythroid and granulocytic differentiation of CD34+ cells from human bone marrow (BM) ex vivo [14].
The objective of this study was to find the effect of total extract of KRG on human BM CD34+ cell proliferation and hematopoietic colony formation.
Go to : 
BM aspirates were obtained from the remnant samples of 8 patients who underwent BM studies for diagnosis after acquiring informed consent. The protocol for this study was approved by the Institutional Review Board of Catholic University hospital of Daegu. Isolation of BM mononuclear cells (MNCs) was performed by following a previously described method [15]. Briefly, 4-6 mL of BM aspirate was mixed with 20 mL of Dulbecco's phosphate-buffered saline (DPBS, Gibco BRL Life technologies, Grand Island, NY). The mixture was resuspended and gently layered onto Ficoll-Hypaque (density, 1.073 g/mL; Gibco BRL Life technologies) and centrifuged at 900 g for 10 min at room temperature. The low-density MNC fraction was collected, combined with 25 mL DPBS, and centrifuged. Two samples were contaminated during cell culture and were therefore excluded from the study.
Cells were resuspended in HSC culture medium (10% fetal bovine serum in Dulbecco's Modified Eagle's medium containing 4.5 g/L glucose (IMDM) with antibiotic/antimycotic supplements (Gibco BRL Life technologies)) and plated at 1×107 cells/100 mm in a culture dish for 90 min. The non-attached cells were then incubated with microbead-conjugated anti-CD34 monoclonal antibody at 4℃ for 20 min and processed through a MiniMACS magnetic separation column (Miltenyi Biotec, GmbH, Bergisch Gladbach, Germany) to obtain the CD34+ cell fraction. After separation, the CD34+ cell count measured by flow cytometry and cell counts determined by the counter were compared. The recovery rate was approximately 100%. This check for the recovery rate was performed only for one sample.
The CD34+ cell preparations isolated by MACS were analyzed by flow cytometry (Cytomics FC 500, Beckmann Coulter, Fullerton, CA) using the single platform CD34 assay kit (Stem-Kit™, Beckman Coulter) to measure the direct counts of CD34+ cells.
The analysis of cell preparations by flow cytometry was carried out using monoclonal antibodies conjugated with fluorescein isothiocyanate (FITC) or phycoerythrine (PE): CD34-PE, CD45-FITC, and isotype-matched controls (Immunotech, Marseille, France). Then, 100 µL of the sample was incubated with the monoclonal antibodies at room temperature in a dark chamber for 20 min. More than 100,000 events were acquired and analyzed by CXP software (Beckman Coulter) [16, 17]. With advanced digital compensation of Cytomics FC 500 (Beckman Coulter), samples could be used for automated color compensation. For manual compensation, users can adjust the slide bars to change the compensation.
The isolated CD34+ cells (5×105) were incubated in IMDM medium, which included HSC-specific medium, H3000 (Stemcell Technology, Vancouver, Canada), StemSpan CC100 (Stemcell Technology), 10% FBS, penicillin/ streptomycin, and L-glutamine, at 37℃ in an atmosphere containing 5% CO2 for 12 days. To compare the expansion ratios, the CD34+ cells from one donor were divided into 3 groups. The CD34+ cells (5×105) were incubated in 3 types of media (HSC-specific medium alone, HSC+100 µg/mL of total extract of KRG (Korean ginseng company, Daejeon, Korea), and 100 µg/mL of total extract of KRG alone for 48 hr), and the number of CD34+ cells was analyzed after liquid culture for 48 hr to determine the effect of media on cell expansion.
To determine the effect of total extract of KRG on hematopoietic colony formation in vitro, colony forming assays were performed using methylcellulose semisolid medium. The expanded CD34+ cells (final concentration: 500 cells per well) were mixed with semisolid medium (MethoCult, Stemcell Technology). Then, the cell-medium mixture was incubated in a 24-well plate for 14 days in medium containing 2% FBS, 50 µg/mL of total extract of KRG, and 100 µg/mL of Ginsenoside Rg1 (Sigma, St. Louise, MO) at 37℃ in an atmosphere containing 5% CO2 and 95% humidity. The hematopoietic colonies with more than 50 cells per group were counted under a microscope [18].
Go to : 
The numbers of BM MNCs from 6 donors were variable (5.6×107, 1.3-48×107, N=6). The numbers of CD34+ cells measured by the single platform flow cytometric method after MACS isolation were also variable among donors (8.9×104, 1.8-80×104, N=5). Therefore, CD34+ cells were 0.19% of total MNCs in the human BM.
The cells were incubated with HSC-specific medium (StemSpan+antimicrobials, L-glutamine, cocktail 100×, IMDM, FBS) for 12 days, and the expansion of CD34+ cells was 40 to 1,944 times the number of CD34+ cells at the beginning of incubation (Table 1).
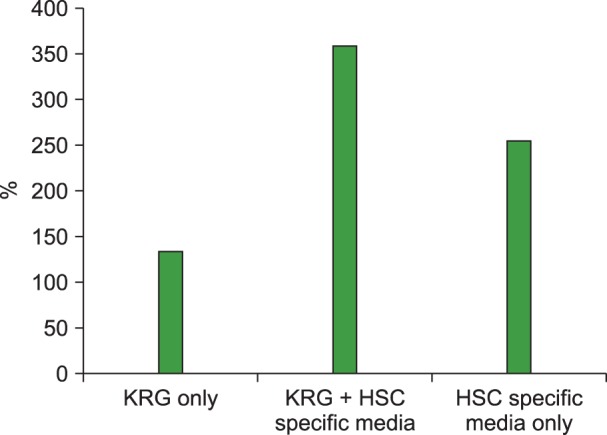
The CD34+ cells (N=1) incubated for 48 hr in medium with 100 µg/mL of total extract of KRG were expanded to 360% (1.8×106). Cells in HSC-specific medium alone were expanded to 255% (1.275×106) and cells in KRG alone expanded to 135% (Fig. 1). Data were normalized to the cell count in media without ginseng extract or Rg1 (100%).
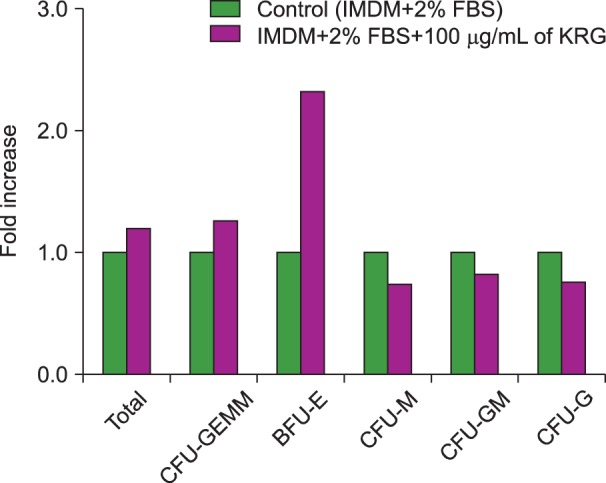
The HSC colonies from 3 BM samples were counted after 14 days of semisolid medium incubation. The total HSC colony numbers were higher in medium with 50 µg/mL KRG when compared to medium with 100 µg/mL KRG, 100 µg/mL Rg1, and conventional medium (Fig. 2). There were more erythroid colonies, burst forming unit-erythroid (BFU-E) and colony forming unit-erythroid (CFU-E), than hematopoietic colonies, colony forming unit-megakaryocyte (CFU-M), colony forming unit-granulocyte-macrophage (CFU-GM), and colony forming unit-granulocyte (CFU-G). The number of CFU-M colonies was the same in medium with or without KRG. The number of CFU-GM and CFU-G colonies was lower in KRG medium (Fig. 3). Rg1 increased the number of CFU-GM and CFU-G colonies, whereas total extract of KRG increased the number of erythroid colonies.
Go to : 
The objective of this study was to determine the effect of total extract of KRG on human BM HSCs. The pure extract of KRG is a highly valued traditional medicine in Korea. The results showed that 50 µg/mL of total extract of KRG increased the number of CD34+ cells when it was added to HSC-specific medium, and it facilitated BFU-E and CFU-E formation, as shown by the methylcellulose assay. Compared to the conventional medium, Rg1, a component of KRG, did not increase the formation of hematopoietic colonies; however, it increased CFU-E, CFU-GM, and CFU-G colony formation. The number of isolated MNCs varied among donors. The number of CD34+ cells after in vitro expansion was also variable among donors. The expansion ratio range was 40 to 1,944.
CD34 is a marker of HSCs. The CD34+ cell count is a very important indicator when collecting stem cells for PBSCT in a clinical laboratory [1, 2]. Therefore, obtaining the exact count of CD34+ cells is mandatory. We used the single platform assay to measure the direct counts of CD34+ cells in the sample. Many groups use the dual platform assay, which initially measures the percentage of CD34+ cells and then calculates the number based on a total cell count. We used the single platform assay to provide more direct and accurate CD34+ cell counts.
Total extract of KRG facilitated the formation of erythroid colonies (BFU-E and CFU-E) in our experiment. The results were in accordance with those of a recent report that TSPG promotes the erythroid differentiation of human CD34+ cells via the EpoR-mediated JAK2/STAT5 signaling pathway [13], although there was no difference in EpoR mRNA expression by the cells in this study. The increased erythroid colony formation demonstrates that expanded CD34+ cells may be committed to the erythroid lineage.
There were different results obtained using Ginsenoside Rg1 and total extract of KRG. Rg1 increased the numbers of CFU-GM and CFU-G colonies, whereas total extract of KRG increased the numbers of BFU-E and CFU-E colonies. This reflected the results of a previous study, which demonstrated that Rg1 may have hematopoietic growth factor-like efficacy based on its ability to upregulate gene expression of the nuclear transcription factor of the glucocorticoid receptor in hematopoietic cells [12]. Therefore, it is necessary to study further the different effects of each component of KRG on hematopoietic progenitors.
There was a dose-dependent effect of total extract of KRG on hematopoietic cells. While 50 µg/mL of KRG increased the CD34+ cell counts, 100 µg/mL of KRG did not increase the CD34+ cell counts. Additionally, in the hematopoietic colony assay, 50 µg/mL of KRG facilitated erythroid colony formation, whereas 100 µg/mL of KRG did not.
A limitation of this study was that the sample size was not large enough. The donors were also not healthy. We used the remnant samples from patients who underwent BM studies to diagnose their hematologic abnormalities. Therefore, it is necessary to perform a study with more samples, including those from a normal healthy population.
Taken together, 50 µg/mL of total extract of KRG facilitated an increase in HSC counts and colony-forming ability, especially of the erythroid lineage. Supplementation with total extract of KRG may provide a potential method of increasing CD34+ cell numbers and enhancing the clinical engraftment for autologous or allogeneic PBSCs in patients with hematologic malignancies.
Go to : 
Notes
This study was supported by grant of Korea Ministry of Health & Welfare, Republic of Korea (Project No.: 090-091-3000-3033-320).
Go to : 
References
1. Specchia G, Pastore D, Mestice A, et al. Early and long-term engraftment after autologous peripheral stem cell transplantation in acute myeloid leukemia patients. Acta Haematol. 2006; 116:229–237. PMID: 17119322.

2. Nielsen JS, McNagny KM. CD34 is a key regulator of hematopoietic stem cell trafficking to bone marrow and mast cell progenitor trafficking in the periphery. Microcirculation. 2009; 16:487–496. PMID: 19479621.

3. Nakamura R, Bahceci E, Read EJ, et al. Transplant dose of CD34(+) and CD3(+) cells predicts outcome in patients with haematological malignancies undergoing T cell-depleted peripheral blood stem cell transplants with delayed donor lymphocyte add-back. Br J Haematol. 2001; 115:95–104. PMID: 11722418.

4. Ergene U, Cagirgan S, Pehlivan M, Yilmaz M, Tombuloglu M. Factors influencing engraftment in autologous peripheral hematopoetic stem cell transplantation (PBSCT). Transfus Apher Sci. 2007; 36:23–29. PMID: 17292672.

5. Papajik T, Pikalova Z, Raida L, et al. Rituximab does not adversely affect the stem cell mobilization and engraftment after high-dose therapy and autologous transplantation in patients with diffuse large B-cell lymphoma in first complete or partial remission. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2009; 153:211–214. PMID: 19851434.

6. Keane C, Gibbs S, Seymour JF, et al. The Hyper-CVAD chemotherapy regimen has an adverse long-term impact on the ability to mobilize peripheral blood stem cells, which can be readily circumvented by using the early cycles for mobilization. Hematol Oncol. 2006; 24:159–163. PMID: 16775841.

7. Yun TK. Experimental and epidemiological evidence on non-organ specific cancer preventive effect of Korean ginseng and identification of active compounds. Mutat Res. 2003; 523-524:63–74. PMID: 12628504.

9. Chen S, Liu J, Liu X, et al. Panax notoginseng saponins inhibit ischemia-induced apoptosis by activating PI3K/Akt pathway in cardiomyocytes. J Ethnopharmacol. 2011; 137:263–270. PMID: 21619920.

10. Kim HD, Ha SE, Kang JR, Park JK. Effect of Korean red ginseng extract on cell death responses in peroxynitrite-treated keratinocytes. J Ginseng Res. 2010; 34:205–211.

11. Kim EH, Lee MJ, Kim IH, Pyo S, Choi KT, Rhee DK. Anti-apoptotic effects of red ginseng on oxidative stress induced by hydrogen peroxide in SK-N-SH cells. J Ginseng Res. 2010; 34:138–144.

12. Gao RL, Chen XH, Lin XJ, Qian XD, Xu WH, Chong BH. Effects of notoginosides on proliferation and upregulation of GR nuclear transcription factor in hematopoietic cells. Acta Pharmacol Sin. 2007; 28:703–711. PMID: 17439727.

13. Chen D, Zuo G, Li C, et al. Total saponins of Panax ginseng (TSPG) promote erythroid differentiation of human CD34+ cells via EpoR-mediated JAK2/STAT5 signaling pathway. J Ethnopharmacol. 2009; 126:215–220. PMID: 19735711.

14. Wang JW, Wang YP, Wang SL, Jiang R. Synergistic effects of total saponins of panax ginseng in combination with hematopoietic growth factor on proliferation and differentiation of CD34(+) cells ex vivo. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2006; 14:959–963. PMID: 17096897.
15. Kim SG, Jeon CH, Suh HS, Choe JY, Shin IH. P-glycoprotein expression in extracellular matrix formation of chondrogenic differentiation of human adult stem cells. Cell Biol Int. 2007; 31:1042–1048. PMID: 17468018.

16. Marinov I, Luxova A, Tkacova V, Gasova Z, Pohlreich D, Cetkovsky P. Comparison of three single platform methods for CD34+ hematopoietic stem cell enumeration by flow cytometry. Clin Lab. 2011; 57:1031–1035. PMID: 22239039.
17. Ngoma A, Saito S, Ohto H, et al. CD34+ cell enumeration by flow cytometry: a comparison of systems and methodologies. Arch Pathol Lab Med. 2011; 135:909–914. PMID: 21732782.

18. Kim SG, Suh HS, Lee JW, et al. Optimization of retrovirus mediated-gene transfer into hematopoietic stem cells. Korean J Biotechnol Bioeng. 1999; 14:593–599.
Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download