Abstract
Background
Although adding rituximab to the chemotherapy regimen of cyclophosphamide, vincristine, doxorubicin, and prednisone (R-CHOP) has improved clinical outcomes of patients with diffuse large B-cell lymphoma (DLBCL), several recent studies have shown that the effect of rituximab is dominantly in the non-germinal center (non-GC) subtype compared to the germinal center (GC) subtype. Natural killer (NK) cell count, a surrogate marker of immune status, is associated with clinical outcomes in DLBCL patients in the rituximab era. We investigated whether the impact of NK cells on clinical outcomes differed according to the immunophenotype of DLBCL.
Methods
This study analyzed 72 DLBCL patients treated with R-CHOP between January 2010 and January 2014.
Results
Low NK cell counts (<100/µL) were associated with poor progression-free survival (PFS) and overall survival (OS) compared to high NK cell counts. In multivariate analysis, low NK cell count was an independent prognostic factor for PFS and OS. However, survival did not significantly differ between the GC and non-GC subtypes. We examined the clinical influence of NK cells according to the immunophenotype and found that low NK cell counts were significantly associated with poor PFS and OS in non-GC cases, but not in GC cases.
Diffuse large B-cell lymphoma (DLBCL) is a malignancy of B-cells and is the most common subtype of non-Hodgkin lymphoma (NHL) in adults. DLBCL is divided into a germinal center (GC) subtype and a non-germinal center (non-GC) subtype according to the immunophenotype [1]. It is known that the GC subtype is associated with better outcomes than the non-GC subtype in DLBCL patients treated with chemotherapeutic agents including cyclophosphamide, vincristine, doxorubicin, and prednisone (CHOP) [2, 3]. Recently, the addition of rituximab to CHOP (R-CHOP) has markedly improved the clinical outcomes of DLBCL patients compared to those treated with CHOP alone [4, 5]. Because of this finding, the R-CHOP regimen is now considered to be the standard treatment for newly diagnosed DLBCL patients. However, some studies have reported that rituximab was effective only in the non-GC subtype but not in the GC subtype. In fact, many studies have reported that there was no difference in clinical outcomes between the GC and non-GC subtypes in patients treated with R-CHOP. This indicates that the anti-tumor activity of rituximab is associated with some factor of the non-GC subtype. Many hematologists have been trying to identify such a factor. We focused on peripheral natural killer (NK) cells because the mechanism of rituximab is known to involve recruitment of NK cells and malignant B-cell lysis via CD16. In 2007, Plonquet et al. [6] reported that the peripheral blood NK cell count was associated with clinical outcomes of DLBCL patients with age-adjusted International Prognostic Index (aaIPI) scores of 2 or 3. Despite many reports on the interactions between the immune system and lymphoma, NK cell research is not very well established. The objective of this study was to investigate whether NK cell counts are associated with clinical outcomes in the rituximab era and to determine whether the impact of NK cell count on survival differs according to the DLBCL immunophenotype.
We retrospectively analyzed 72 newly diagnosed DLBCL patients. Patients were recruited from the Pusan National University Hospital and treated with R-CHOP as a first-line therapy between January 2010 and January 2014. Patients were included if they were newly diagnosed with DLBCL after histological analysis, and if data for immunohistochemical staining and T/NK cell subset measures were available at diagnosis. Exclusion criteria included previous receipt of other treatments such as autologous stem cell transplantation, chemotherapy with something other than R-CHOP, or radiotherapy. Patients were also excluded if they were experiencing conditions affecting their immune system, such as human immunodeficiency virus infection or autoimmune disease, or if they showed other evidence of infection at diagnosis. The International Prognostic Index (IPI) includes the stage, serum lactate dehydrogenase (LDH) level, Eastern Cooperative Oncology Group (ECOG) performance status, and the number of extranodal sites. Each patient was evaluated using this clinical tool for prognosis. All patients underwent a staging investigation according to the Ann Arbor staging system including accessible lymph nodal or extranodal biopsy, computed tomography, and bone marrow aspiration and biopsy. Furthermore, the presence of B symptoms and bulky disease were investigated. We denoted high IPI scores as IPI score >3. There were 16 patients (22.2%) with high IPI scores and 56 patients (77.8%) with low IPI scores (Table 1). A written statement of informed consent was obtained from each patient.
All patients were scheduled to receive 6 to 8 cycles of R-CHOP therapy. If a patient experienced a chemotherapy-induced toxicity greater than grade 3, the doses of the CHOP agents were reduced to 75%, but the dose of rituximab was not reduced. Response was assessed every 3 cycles during R-CHOP treatment and classified as complete response, partial response, stable disease, and progressive disease according to the International Workshop Criteria. In the case of relapse, a second-line therapy was provided with etoposide, methylprednisolone, cytarabine, and cisplatin (the ESHAP regimen).
Sections from formalin-fixed paraffin blocks were transferred to poly-L-lysine-coated glass slides and air-dried overnight at 37℃. Sections were then deparaffinized in xylene (3 times), rehydrated in a graded series of decreasing ethanol concentrations, and rinsed in Tris-buffered saline (pH 7.4). Endogenous peroxidase activity was inactivated with 5% hydrogen peroxide in methanol for 15 min at 37℃. Heat-induced antigen retrieval was carried out for 45 min in Tris-ethylenediaminetetraacetic acid buffer (pH 8) in a pressure cooker at 95℃ for the following 4 antibodies: Bcl-6 (clone PGB6P, working dilution 1:40; Dako, Copenhagen, Denmark), CD10 (clone 56C6, working dilution 1:200; Lab Vision Corp., Fremont, CA, USA), and MUM-1 (clone MUM1p, working dilution 1:50; Dako, Copenhagen, Denmark). Next, the primary antibodies were incubated for 1 h at room temperature. The antibody in an EnVision™ Chem™ Detection Kit (Dako, Carpinteria, CA, USA) was used for the secondary antibody at room temperature for 30 min; 3,3'-diaminobenzidine was used as a chromogen, and then Mayer hematoxylin counterstain was applied. Immunohistochemical reactions were evaluated semiquantitatively by considering the percentage of positive tumor cells in the neoplastic tissue. In agreement with Hans et al. [1, 8], the optimal cut-off value was considered to be 30% for CD10, Bcl-6, and MUM-1. The samples were analyzed independently by 2 pathologists, and disagreements were resolved by a joint review after observing the samples with a multi-head microscope. GC and non-GC subtypes were determined based on CD10, Bcl-6, and MUM-1 staining according to an algorithm developed by Hans et al. [8]. The GC subtype was defined as CD10+ or CD10-/Bcl-6+/MUM-1-. The non-GC subtype was defined as CD10-/Bcl-6- or CD10-/Bcl-6+/MUM-1+.
Serum T/NK cell subsets (CD4+ and CD8+ T-cells, B-cells, and NK cells) were counted for all patients at diagnosis. Peripheral lymphocytes were stained with immunofluorescent antibodies available from Beckman-Coulter and subsequently analyzed by flow cytometry (Navios flow cytometer; Beckman-Coulter, Brea, CA, USA). Lymphocytes were gated according to high-CD45 fluorescence intensity and low side scatter intensity; B cells were defined as CD19+ lymphocytes, CD4 T-cells were CD3+CD4+ lymphocytes, CD8 T-cells were CD3+CD8+ lymphocytes, and NK cells were CD3-CD16+ and/or CD56+ lymphocytes [6]. Absolute NK cell counts were collected and classified as being high and low NK cell counts using a cut-off value of 100 cells/µL.
Chi-squared tests were used to assess differences in clinical outcomes according to prognostic factors. Progression-free survival (PFS) was calculated from the date of diagnosis to the date of relapse. Overall survival (OS) was calculated from the date of diagnosis until death from any cause or the latest date known to be alive. PFS and OS were estimated using the Kaplan-Meier method. Statistical data processing was performed using SPSS software, version 18.0 (SPSS Inc., Chicago, IL, USA). P values <0.05 were considered statistically significant.
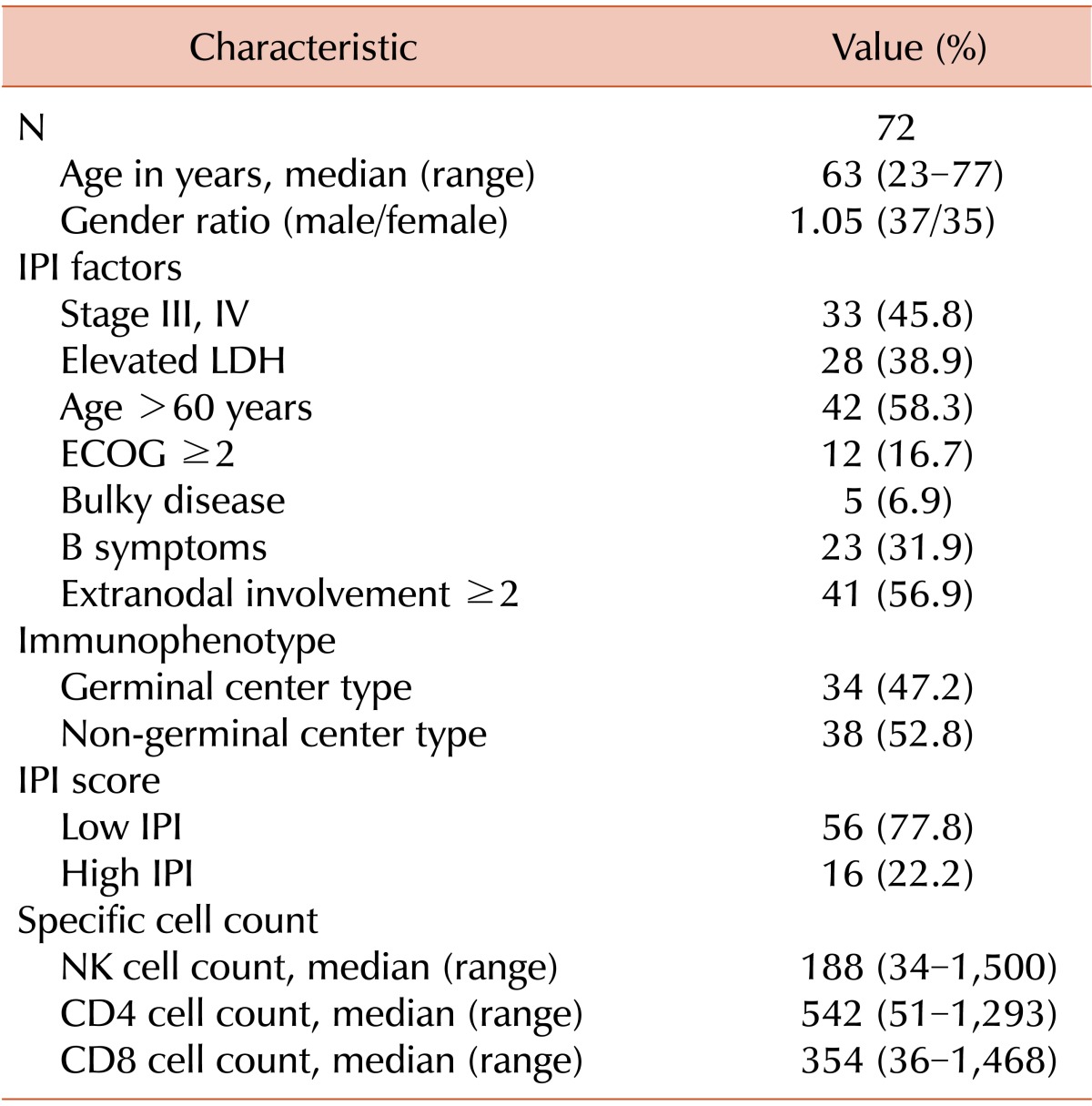
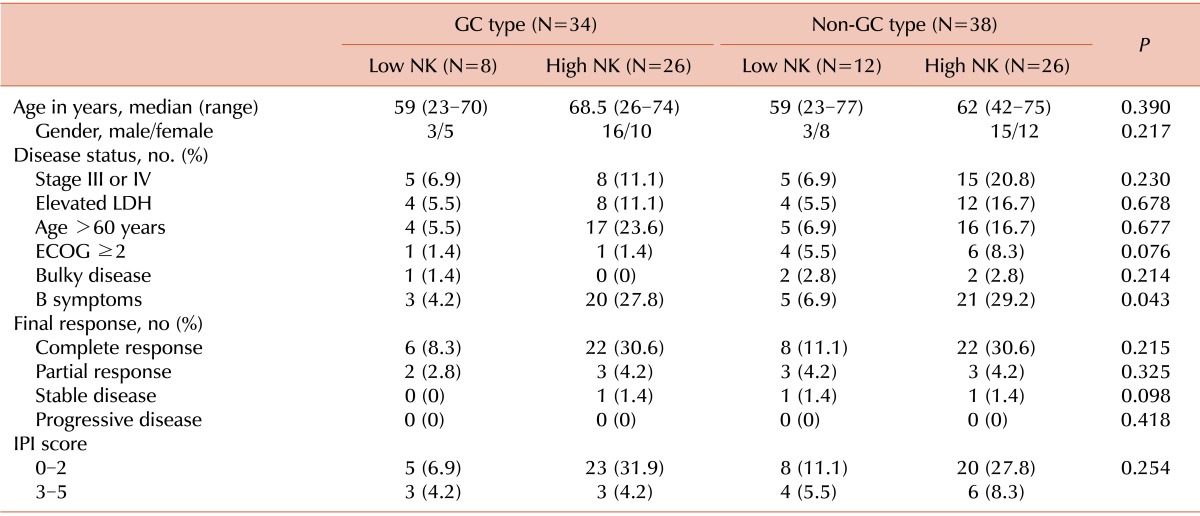
This study included 72 patients treated with R-CHOP as a first-line therapy between January 2010 and January 2014. The median age was 63 years (range, 23-77 years), and 42 patients (58.3%) were >60 years. The male-to-female ratio was 1.05 (37 males, 35 females). Five patients received radiotherapy after R-CHOP, and the amount of absorbed radiation was 45 to 50 Gy. Thirty-three patients had advanced stage disease (stages III and IV), and 28 patients had elevated LDH levels. In addition, 12 patients had a poor performance status (ECOG 3 or 4), and 23 patients had a B symptom at diagnosis. Only 5 patients had bulky disease, and 41 patients had extranodal involvement at more than 2 sites, including the stomach, bowel tract, lungs, and liver. There were 34 cases of GC subtype disease and 38 cases of non-GC subtype disease (47.2% vs. 52.8%, respectively). Different cut-off levels were analyzed using a log-rank test to examine values between the 25% and 75% quartiles. This analysis determined that setting the cut-off point at 1.0×109 NK cells/L yielded the highest difference in OS and PFS. Thus, this cut-off level was used for statistical analysis. The median NK cell count was 188 cells/µL (range, 3-1,500 cells/µL). The baseline characteristics of the patients are described in Table 1. With a cut-off value of 100 NK cells/µL, 20 patients had a low NK cell count, while the other 52 patients had a high NK cell count at diagnosis. Among the 20 patients with low NK cell counts, 8 patients had GC subtype DLBCL and 12 patients had non-GC subtype DLBCL. Alternatively, among the 52 patients with high NK cell counts, 26 patients had GC subtype DLBCL and 26 patients had non-GC subtype DLBCL (Table 2). Several prognostic factors were compared among the 4 subgroups formed based on the immunophenotype and NK cell count (GC subtype with high NK, GC subtype with low NK, non-GC subtype with high NK, and non-GC subtype with low NK). The only significant difference observed among the 4 subgroups was in B symptoms (Table 2). The baseline characteristics were well balanced.
The overall response rate (ORR) was 95.8% (69 of 72 patients) with a CR rate of 80.5% (58 of 72 patients) and a PR rate of 15.2% (11 of 72 patients). There was no significant difference in ORR (CR+PR) when the groups with different immunophenotypes were compared (97% in GC type vs. 95% in non-GC type). Additionally, there was no significant difference in ORR between low NK cell and high NK cell groups (95% in low NK vs. 96% in high NK). The NK cell count did not influence treatment response, as measured by ORR, in each subtype. Most patients achieved a good response after R-CHOP regardless of their immunophenotype and NK cell count. However, there was no statistical difference (Table 2).
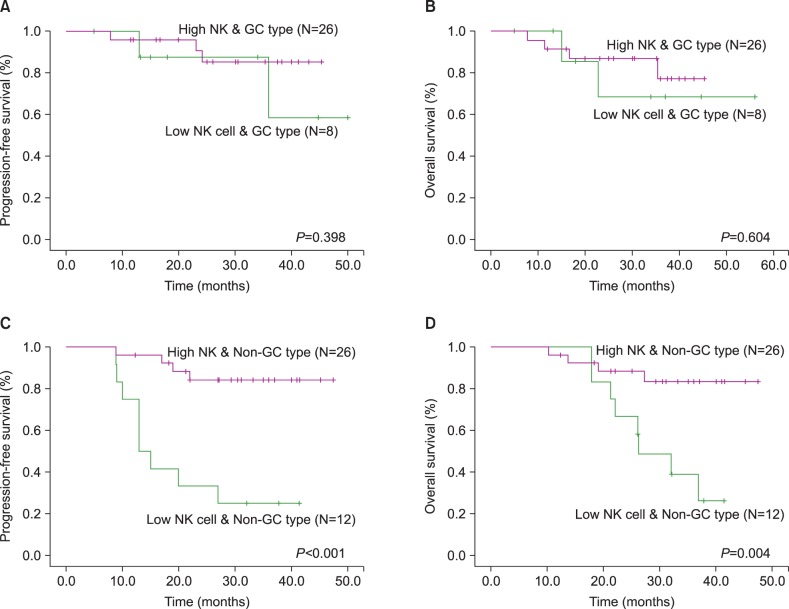
We analyzed clinical outcomes according to immunophenotype. Although the non-GC subtype showed a trend toward poor PFS and OS, there was no significant difference between the 2 subgroups (Fig. 1A and 1B). However, analysis of outcomes according to NK cell count revealed a significant difference between the 2 subgroups (Fig. 1C and 1D). Low NK cell count was associated with poor PFS and OS (P<0.001 and P=0.003, respectively). We also investigated the impact of NK cells on survival according to immunophenotype. In GC subtype patients, there was no significant difference in outcomes between patients with high NK cell counts and low NK cell counts. However, in non-GC subtype patients, low NK cell counts were associated with poor PFS and OS (P<0.001 and P=0.004, respectively) (Fig. 2C and 2D).
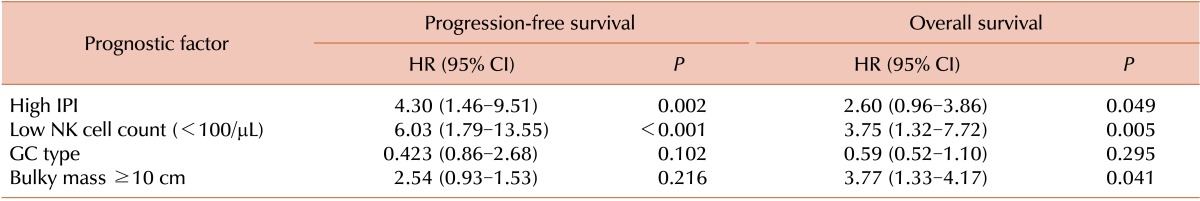
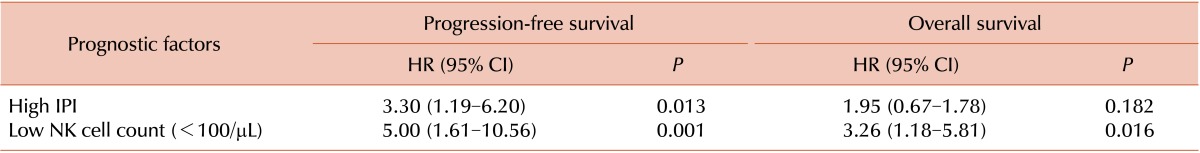
Univariate analysis revealed that individuals with high IPI scores (IPI score ≥3) and low NK cell counts had lower PFS (high IPI score: hazard ratio [HR]=4.30, P=0.002; low NK cell count: HR=6.03, P<0.001) and OS (high IPI score: HR=2.60, P=0.049; low NK cell count: HR=3.75, P=0.005) (Table 3). Multivariate analysis revealed that patients with low NK cell counts had lower PFS (HR=5.0, P=0.001) and OS (HR=3.26, P=0.016), independent of IPI score (Table 4).
DLBCL is a malignancy of B-cells, a type of white blood cell responsible for producing antibodies, and is the most common subtype of NHL in adults [7]. The subtypes of DLBCL are defined by means of gene expression profiling [8]. The worldwide incidence of NHL is rising. The annual incidence of NHL is estimated to be 15 to 20 cases per 100,000 individuals in Europe and the USA [9], and DLBCL accounts for approximately 31% of all NHL in Western countries [10]. The etiology of DLBCL remains unknown. In the 2008 World Health Organization (WHO) Classification of Tumors of the Hematopoietic and Lymphoid Tissues, DLBCL categories are described based on morphological, immunohistochemical, and molecular patterns [11]. In this classification, DLBCL is dichotomized into 2 subgroups based on immunohistochemical analysis: GC B-cell-like and non-GC B-cell-like. Although DLBCL has a poor prognosis, with a median survival of <1 year in untreated patients [12], patients can be cured with combination chemotherapy such as CHOP, which has been the mainstay of therapy for several decades. Because DLBCL is biologically heterogeneous, a number of studies have suggested that novel combinations of chemotherapy or immunotherapy may improve survival. The addition of rituximab, an anti-CD20 monoclonal antibody, to CHOP chemotherapy led to markedly improved outcomes compared to CHOP alone [4, 5]. R-CHOP has now become the first-line standard treatment for DLBCL. Prognostic factors for DLBCL include factors related to the patient, the tumor itself, aggressiveness indicators, and the therapeutic strategy. The IPI and aaIPI have been widely used as models for predicting outcomes based on clinical factors. However, the prognosis of patients with DLBCL varies depending on their biological parameters; this is especially true in the rituximab era. Molecular signatures identified through gene expression profiling can provide further information about disease prognosis, and this could become the foundation for novel therapeutic targets and agents for DLBCL.
We investigated the therapeutic outcomes in patients with GC versus non-GC subtype DLBCL treated with R-CHOP. The GC and non-GC subtypes were defined according to the Hans algorithm [8]. In this study, the PFS and OS were not significantly different between the 2 subtypes. These results are similar to previous studies that found that the Hans algorithm did not have prognostic value in patients with DLBCL treated with R-CHOP [13]. Recent studies showed that a low absolute leukocyte count (ALC), which is a surrogate marker of host immune status, at diagnosis was associated with poor outcomes in DLBCL patients treated with CHOP or R-CHOP [14, 15, 16]. This indicates that ALC at diagnosis might affect clinical outcome. However, another study showed conflicting results; low NK cell count, but not ALC, was a negative prognostic marker for event-free survival [6]. In the current study, we focused on NK cells based on the relationship between their molecular properties and the mechanism of rituximab. We compared therapeutic outcomes according to the peripheral NK cell count at diagnosis in DLBCL patients treated with R-CHOP. Our results showed that a low NK cell count was associated with poor PFS and OS in DLBCL patients who received R-CHOP therapy. We suggest that this may be due to the mechanism of rituximab in malignant lymphoma. Rituximab is known to have anti-tumor activity through 4 signaling pathways: antibody-dependent cell-mediated cytotoxicity (ADCC), complement-dependent cytotoxicity, direct signaling triggering apoptosis, and increased sensitivity to chemotherapy [17]. Among these pathways, the ADCC signaling pathway predominantly affects anti-tumor activity. In this signaling pathway, rituximab recruits NK cells towards malignant B cells via CD16, and the NK cells subsequently kill the malignant cells [18]. Therefore, it is easily assumed that higher NK cell counts are associated with rituximab-induced cytotoxicity. Additionally, we investigated whether there was a correlation between NK cell count and rituximab-induced cytotoxicity. The present study demonstrated that low NK cell counts were associated with poor outcomes only in non-GC subtype DLBCL. Our data shows that, in the rituximab era, the absolute number of NK cells is influential in the non-GC subtype, but not in the GC subtype. Previous studies have seen similar results [19, 20]. We propose that these results might be associated with the mechanism of rituximab. Rituximab presumably acts by inhibiting the activity of inhibitor of κB kinase, leading to consequent down-regulation of nuclear factor κB (NF-κB), a protein that increases cancer cell resistance to apoptosis [21]. Rituximab leads to increased sensitivity to chemotherapy through this mechanism. In addition, the NF-κB levels are lower in the GC subtype compared to the non-GC subtype. Therefore, it makes sense theoretically that higher NK cell counts and rituximab-induced cytotoxicity are more associated with the non-GC subtype.
There are some limitations in the present study. The first limitation is its retrospective nature and small sample size. Only 72 patients were analyzed in the present study, and this population included only 38 cases of non-GC DLBCL for the study of statistical associations with outcomes. The sample size was small because the NK/T cell subset laboratory tests were not performed in all DLBCL patients treated with R-CHOP. Further large-scale prospective studies are necessary to strengthen our conclusions.
A second limitation is that the DLBCL subtypes were determined only according to the Hans algorithm using brief immunohistochemical staining. Though the Hans algorithm is very convenient and simple, it has come to be controversial in recent years. New technologies, such as gene expression profiling, RNA interference screening, and DNA sequencing, are being studied for their ability to identify subtypes of DLBCL [22]. Unfortunately, molecular techniques were not applied to define the subtypes of DLBCL in our study. More precise classification methods are necessary in future research.
A third limitation is that most of patients with high NK cell counts (83%) had low IPI scores, and most patients with low NK cell counts (65%) had high IPI scores. IPI scores could have impacted the clinical finding that low NK cell count was associated with poor PFS and OS in this study. However, Plonquet et al. [6] reported that peripheral blood NK cell counts were associated with clinical outcomes. Moreover, the mechanism of rituximab in the field of molecular biology supports that fact that NK cell count was associated with clinical outcomes [18]. Therefore, our data suggests that NK cell count was a significant prognostic factor in the rituximab era. However, in future studies, IPI scores should be balanced in each NK cell count group.
In conclusion, we found that low NK cell counts at diagnosis were associated with poor outcomes in patients with non-GC subtype DLBCL treated with R-CHOP. Therefore, the peripheral NK cell count before R-CHOP might be a significant prognostic factor in patients with non-GC subtype DLBCL. Furthermore, this could become the foundation for development of new therapeutic agents targeting the activation of NK cells. A future well-designed prospective study is necessary to further explore these possibilities.
References
1. Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004; 103:275–282. PMID: 14504078.

2. Berglund M, Thunberg U, Amini RM, et al. Evaluation of immunophenotype in diffuse large B-cell lymphoma and its impact on prognosis. Mod Pathol. 2005; 18:1113–1120. PMID: 15920553.

3. van Imhoff GW, Boerma EJ, van der Holt B, et al. Prognostic impact of germinal center-associated proteins and chromosomal breakpoints in poor-risk diffuse large B-cell lymphoma. J Clin Oncol. 2006; 24:4135–4142. PMID: 16943530.

4. Feugier P, Van Hoof A, Sebban C, et al. Long-term results of the R-CHOP study in the treatment of elderly patients with diffuse large B-cell lymphoma: a study by the Groupe d'Etude des Lymphomes de l'Adulte. J Clin Oncol. 2005; 23:4117–4126. PMID: 15867204.

5. Pfreundschuh M, Trumper L, Osterborg A, et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol. 2006; 7:379–391. PMID: 16648042.
6. Plonquet A, Haioun C, Jais JP, et al. Peripheral blood natural killer cell count is associated with clinical outcome in patients with aaIPI 2-3 diffuse large B-cell lymphoma. Ann Oncol. 2007; 18:1209–1215. PMID: 17496307.

7. Martelli M, Ferreri AJ, Agostinelli C, Di Rocco A, Pfreundschuh M, Pileri SA. Diffuse large B-cell lymphoma. Crit Rev Oncol Hematol. 2013; 87:146–171. PMID: 23375551.

8. Reber R, Banz Y, Garamvolgyi E, Perren A, Novak U. Determination of the molecular subtypes of diffuse large B-cell lymphomas using immunohistochemistry: a case series from the Inselspital, Bern, and a critical appraisal of this determination in Switzerland. Swiss Med Wkly. 2013; 143:w13748. PMID: 23740154.

9. Fisher SG, Fisher RI. The epidemiology of non-Hodgkin's lymphoma. Oncogene. 2004; 23:6524–6534. PMID: 15322522.

10. The Non-Hodgkin's Lymphoma Classification Project. A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkins lymphoma. Blood. 1997; 89:3909–3918. PMID: 9166827.
11. Campo E, Swerdlow SH, Harris NL, Pileri S, Stein H, Jaffe ES. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical application. Blood. 2011; 117:5019–5032. PMID: 21300984.
12. Flowers CR, Sinha R, Vose JM. Improving outcomes for patients with diffuse large B-cell lymphoma. CA Cancer J Clin. 2010; 60:393–408. PMID: 21030533.

13. Castillo JJ, Beltran BE, Song MK, et al. The Hans algorithm is not prognostic in patients with diffuse large B-cell lymphoma treated with R-CHOP. Leuk Res. 2012; 36:413–417. PMID: 22277681.

14. Kim DH, Baek JH, Chae YS, et al. Absolute lymphocyte counts predicts response to chemotherapy and survival in diffuse large B-cell lymphoma. Leukemia. 2007; 21:2227–2230. PMID: 17554383.

15. Cox MC, Nofroni I, Laverde G, et al. Absolute lymphocyte count is a prognostic factor in diffuse large B-cell lymphoma. Br J Haematol. 2008; 141:265–268. PMID: 18353165.

16. Oki Y, Yamamoto K, Kato H, et al. Low absolute lymphocyte count is a poor prognostic marker in patients with diffuse large B-cell lymphoma and suggests patients' survival benefit from rituximab. Eur J Haematol. 2008; 81:448–453. PMID: 18691256.

17. Plosker GL, Figgitt DP. Rituximab: a review of its use in non-Hodgkin's lymphoma and chronic lymphocytic leukaemia. Drugs. 2003; 63:803–843. PMID: 12662126.
19. Nyman H, Adde M, Karjalainen-Lindsberg ML, et al. Prognostic impact of immunohistochemically defined germinal center phenotype in diffuse large B-cell lymphoma patients treated with immunochemotherapy. Blood. 2007; 109:4930–4935. PMID: 17299093.

20. Saito B, Shiozawa E, Usui T, et al. Rituximab with chemotherapy improves survival of non-germinal center type untreated diffuse large B-cell lymphoma. Leukemia. 2007; 21:2563–2566. PMID: 17597802.

21. Pavan A, Spina M, Canzonieri V, Sansonno S, Toffoli G, De Re V. Recent prognostic factors in diffuse large B-cell lymphoma indicate NF-kappaB pathway as a target for new therapeutic strategies. Leuk Lymphoma. 2008; 49:2048–2058. PMID: 19021048.
22. Dunleavy K, Wilson WH. Appropriate management of molecular subtypes of diffuse large B-cell lymphoma. Oncology (Williston Park). 2014; 28:326–334. PMID: 24839807.
Fig. 1
Comparison of clinical outcomes according to immunohistochemical staining for germinal center (GC) versus non-GC subtype (A, B) or natural killer (NK) cell counts in the entire population with diffuse large B-cell lymphoma (C, D).
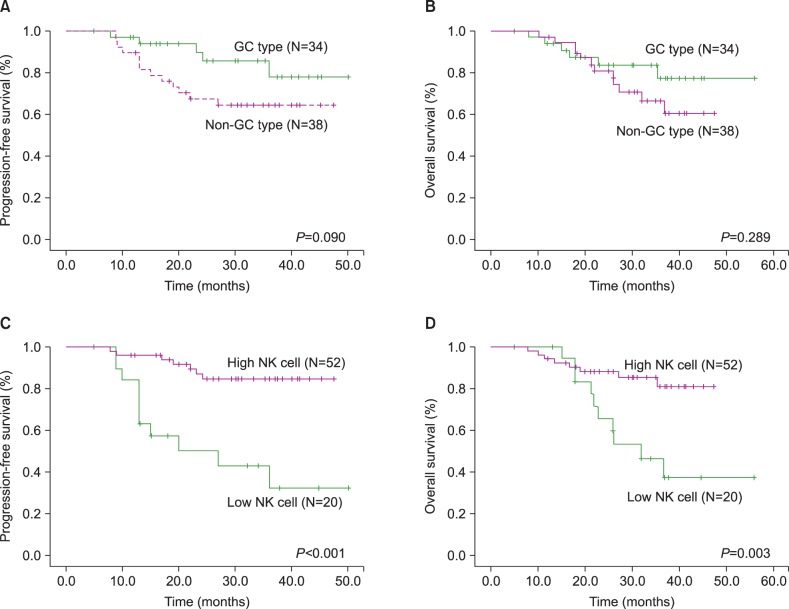
Fig. 2
Comparison of clinical outcomes according to natural killer (NK) cell counts in germinal center (GC) type (A, B) or non-GC type (C, D).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download