INTRODUCTION
Over the past decades, multi-parametric hematology analyzers have evolved to the point of being capable of performing automated white blood cell (WBC) differential counts [
1]. Since their introduction, these analyzers have become progressively more sophisticated and currently they produce superior red blood cell (RBC), WBC, and platelet counts compared with manual methods. The analyzers' WBC differential counts are also superior to the visual microscopic film differential counts for mature cells [
2]. The majority of analyzers, however, are relatively ineffective in the proper recognition of abnormal cells [
3], and therefore the instruments give "flag" messages when such cells are present in the blood. For this reason, manual WBC differential count by microscopy remains the current gold standard [
4,
5].
The manual WBC differential count has several limitations of critical significance to laboratorians, both from a diagnostic and an economic point of view. They are very labor-intensive and time-consuming, especially in severely leukopenic samples. Although it is recommended that at least 200 cells should be counted, often it is not possible to count more than 100 cells in severely leukopenic samples. This makes manual differential results more inaccurate in severely leukopenic samples. Furthermore, as examining the slides of severely leukopenic samples is time consuming, the pressure is increased on laboratories with many severely leukopenic samples [
6]. Performing a manual differential count is particularly cumbersome in severely leukopenic samples with WBC counts below 1,000/µL. The number of such samples has markedly increased in hospital laboratories in recent years, largely due to an increase in the number of patients receiving chemotherapy, radiotherapy, and transplantation [
7,
8]. To add to the challenge, the morphology of WBCs in these samples may be altered due to chemotherapy or radiotherapy, rendering manual differential counts even more difficult. Because of these reasons, manual WBC differentials show higher variability in leukopenic samples [
9,
10].
Recently, a new flow cytometric differential counting method called Hematoflow (Beckman Coulter, Miami, FL, USA) was introduced. This method uses a 5-color/6-antibody reagent cocktail with an auto-gating program [
11], and reports 17 WBC cell populations, including blasts, immature granulocytes, and lymphocyte subsets, which are not reported using automatic hematology analyzers or manual differential counts. We found that this method gave reliable and accurate results in leukopenic samples [
12]. However, it has not been studied in severe leukopenic samples. The objective of this study was to comparatively evaluate the performance of the counting methods mentioned above in severely leukopenic, challenging cases.
Go to :

MATERIALS AND METHODS
Patients and samples
One hundred seventy-five EDTA-anticoagulated blood samples were used in the analysis from 172 patients (96 males and 76 females; age 0-76 years) who had WBC counts of 40-990/µL in routine CBC determined by a Sysmex XE-2100 analyzer (Sysmex, Kobe, Japan). The patients' initial diagnoses were as follows: 57 cases of acute myeloid leukemia (AML), 36 cases of acute lymphoblastic leukemia (ALL), 28 cases of solid tumors, 18 cases of malignant lymphomas, 11 cases of myelodysplastic syndrome (MDS), 9 cases of aplastic anemia, 4 cases of chronic myelogenous leukemia (CML), 3 cases of plasma cell myeloma, 2 cases of mixed phenotype acute leukemia, and 4 cases of other diseases. Four AML samples, 3 ALL samples, and 1 malignant lymphoma sample had blasts on the manual differential. This study was approved by the Institutional Review Board.
Manual differential count
A trained hematology technician who had worked over 20 years in the manual slide review section of a diagnostic hematology laboratory and a senior resident with significant research experience in diagnostic hematology performed the manual WBC differential by counting 10-200 cells. Since the number of cells per slide was too small, we did not analyze the reproducibility of the manual counting method and used the sum of the counts by 2 observers using 2 slides for the manual counts. In some cases, leukocyte morphology was markedly altered, and it was hard to classify such cells. We compared the abundances of 3 main leukocyte subpopulations (neutrophils, lymphocytes, and monocytes) and that of blasts and did not analyze other cell populations due to the scarcity of countable cells. We measured the time required for the manual count of each case, and then average analysis time was calculated.
Hematoflow differential count
Hematoflow differential counts were performed using an FC500 flow cytometer, the CytoDiff reagent, and a flow cytometry analysis software (all from Beckman Coulter) within 4 hours after blood collection. The CytoDiff cocktail included CD36-FITC, CD2-PE, CD294-PE, CD19-ECD, CD16-PC5, and CD45-PC7 antibodies. Leukocytes were differentiated into 17 cell populations (B-lymphocytes, CD16- T-lymphocytes, CD16+ T-lymphocytes, T & NK lymphocytes, total lymphocytes, CD16- monocytes, CD16+ monocytes, total monocytes, immature granulocytes (IGs), total eosinophils, mature neutrophils, total neutrophils, B blasts (Xb), T blasts (Xt), monoblasts (Xm), myeloblasts (Xn), and total basophils). All analysis procedures followed the manufacturer's instructions. In brief, 100 µL of whole blood was mixed with 10 µL of CytoDiff reagent and incubated for 20 min at room temperature. RBCs were disrupted by incubation in a lysis solution (Versalyse solution, Beckman Coulter) for 15 min. Without washing, about 10,000 cells were analyzed using a flow cytometer (FC500) and a 32-tube carousel. Results were analyzed automatically by auto-gating analysis software, which separates populations using a built-in algorithm (
Fig. 1). As instructed by the manufacturer, gates were only adjusted when analyzing samples with excessive debris. We analyzed 175 samples, of these 170 in duplicate, and a total of 345 tests were performed. We measured the time required for each test and then average analysis time was calculated.
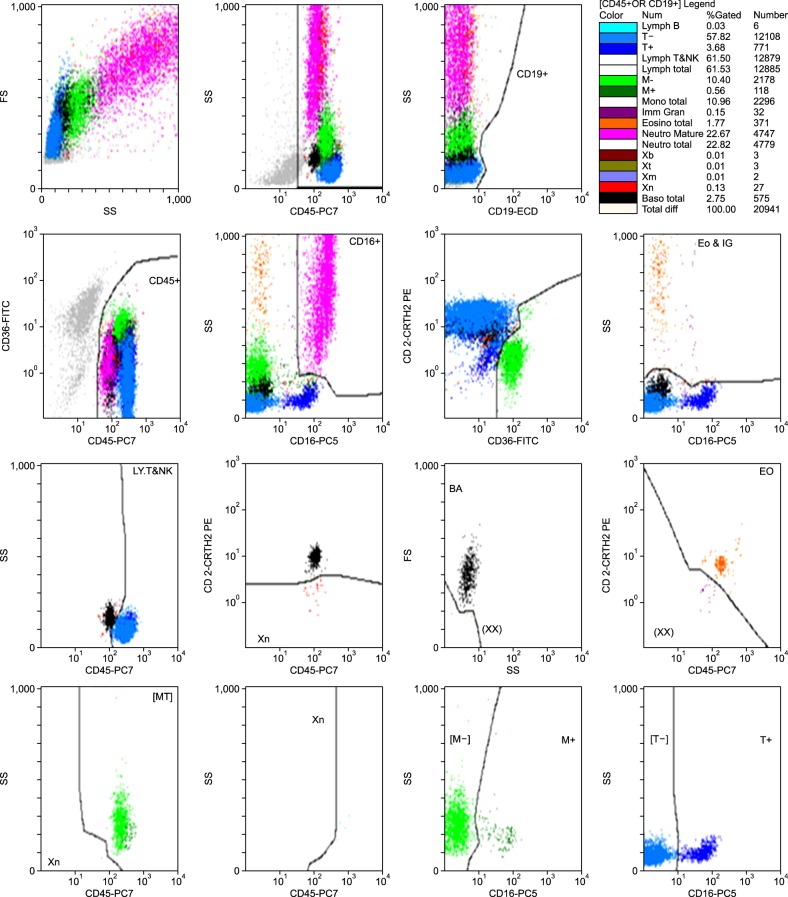 | Fig. 1An example of Hematoflow results. Seventeen cell populations are displayed in different colors using specific gates recommended by the manufacturer. 
|
Differential counts using automated blood cell analyzers
The performance of 2 automated hematology analyzers: DxH 800 (Beckman Coulter, Miami, FL, USA) and XE-2100 were analyzed. For both instruments, the number of samples for which the instrument failed to report any differential count was recorded. Only the differential results for the 3 main leukocyte subtypes (neutrophils, lymphocytes, and monocytes) were analyzed. Basophils and eosinophils were excluded from the analysis, since they are present in extremely low numbers in severely leukopenic samples.
Statistical analysis
The correlation coefficient, standard deviation (SD), and coefficient of variation (CV) between results obtained by each method and the duplicate counts were calculated for leukocyte subpopulations. We used MedCalc v11.2 (Mariakerke, Belgium) to perform the statistical analysis. To show the reproducibility of Hematoflow tests, we used the variance ratio of f-test.
Go to :

RESULTS
Analysis time and gate adjustment
Given the paucity of cells, the average countable WBC number per case was only 54 cells in the manual count. The average time for manual count in each case was 183.7 s. The total time for the manual count of 20 samples was approximately 115 min, including sample acquisition, automated production and staining of blood smears, drying, and manual counting. The total time required for the microscopic examination was approximately 61 min for 20 samples.
The total time required to analyze 20 severely leukopenic samples (10,000 events) by the Hematoflow method was approximately 90 min, including incubation and reading time, and the hands-on time was 15 min. In Hematoflow counting, gates were adjusted in 30 out of 345 tests (8.7%). Adjustments were made only when large debris contamination was present.
The reproducibility of Hematoflow counts
The mean differential neutrophil count in the 170 samples was 25.09% in the first analysis and 24.98% in the second analysis. The standard deviation (SD) for the difference between neutrophil counts was 1.47%, and the coefficient of variation (CV) between both analyses was 5.89%, with a correlation coefficient of 0.99 and a variance ratio of 0.98.
The mean differential lymphocyte count in the 170 samples was 52.41% in the first analysis and 52.38% in the second analysis. The SD for the difference between lymphocyte counts was 1.30%, and the CV between both analyses was 2.49%, with a correlation coefficient of 0.99 and a variance ratio of 0.96.
The mean monocyte count in the 170 samples was 7.46% in the first analysis and 7.74% in the second analysis. The SD for the difference between the monocyte counts was 2.4%, and the CV between both analyses was 31.70%, with a correlation coefficient of 0.95 and a variance ratio of 0.67.
Comparison of differential count results obtained using Hematoflow, automatic blood cell analyzers and manual counts
Neutrophils
In samples with WBC counts less than 100/µL, the XE-2100 instrument did not report the WBC differential. The XE-2100 was able to report a differential count in 85% of the samples included in the study (148 out of 175). The DxH 800 instrument and Hematoflow counting were able to report a differential count in all 175 samples.
The correlation coefficient between Hematoflow and XE-2100 neutrophil counts was 0.900, whereas between Hematoflow and DxH800 counts it was 0.870. The results obtained using the 2 instruments correlated well (r=0.948).
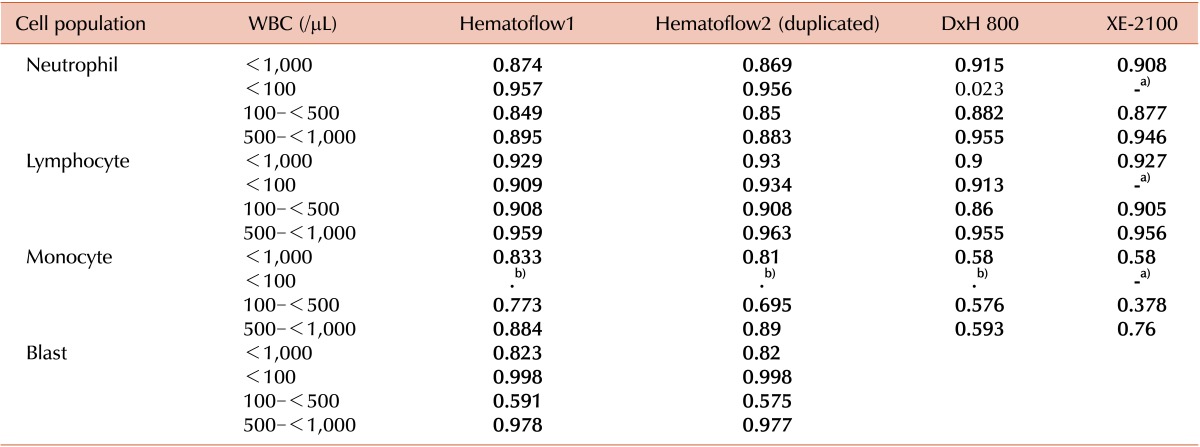
The overall correlation of manual neutrophil counts was good with Hematoflow as well as with DxH 800 and XE-2100 counts (
Table 1). While the correlation between DxH800 and manual neutrophil counts was very poor in samples with WBC counts less than 100/µL, the correlation between manual and Hematoflow counts was much better (
Table 1).
Table 1
Results of Correlation between the results obtained by the Hematoflow method (in duplicates), by automated analyzers and by manual counting. Shown are the correlation coefficients.

Lymphocytes
The correlation coefficient between Hematoflow and XE-2100 lymphocyte counts was 0.950, while between Hematoflow and DxH800 counts it was 0.950. The results obtained using the 2 instruments correlated well (r=0.956). The correlation coefficient between manual and Hematoflow lymphocyte counts was high (r=0.929 and r=0.930), as well as between manual and DxH 800 (r=0.900) and manual and XE-2100 counts (r=0.927). Both Hematoflow and DxH800 lymphocyte counts correlated very well with manual counts in samples with WBC counts less than 100/µL (
Table 1).
Monocytes
The correlation coefficient between Hematoflow and XE-2100 monocyte counts was 0.730, while between Hematoflow and DxH800 counts it was 0.67. The correlation between the results obtained using the 2 instruments was poor (r=0.618). The correlation coefficient between manual and Hematoflow monocyte counts was higher than between Hematoflow and any other monocyte counts (
Table 1). In samples with WBC counts less than 100/µL, we could not count monocytes manually.
Blasts
Neither of the 2 automated instruments (DxH 800 and XE-2100) reported the blast counts. Hematoflow blast counts showed good correlation with manual counts (r=0.823 and r=0.82). The correlation between repeated Hematoflow blast counts was very good (r=0.995,
Table 1). The range of total blast counts was 0.08-89.6%. None of the 154 cases showing less than 4% blasts by the Hematoflow method revealed any blasts in the manual counts. Only 8 of 21 cases (38.1%) showing over 4% blasts by the Hematoflow method had blasts in the manual counts while the remaining cases had no blasts. Of these 13 cases, 5 cases showed debris contamination in the scattergrams. The basophils were counted as blasts in 7 of the 13 cases. Most basophils showed a higher fluorescence than the blasts did after staining with antibodies to CD2 and CD294, but in some of them the fluorescence was under the cutoff level between blasts and basophils and therefore they were counted to the blast population. Some of the monocytes were counted as monoblasts in 1 case due to similar reasons.
Go to :

DISCUSSION
The absolute neutrophil count (ANC) or the presence of blasts is an important criterion to clinicians when making decisions about the treatment of patients [
13,
14,
15]. However, because many severely leukopenic patients receive chemotherapy and/or radiotherapy, the morphology of cells may be altered [
16]. Therefore, performing a manual differential count could be more difficult in severely leukopenic samples. Manual differential counting requires the preparation of blood smears, which involves fixation, staining, washing, and drying, and microscopic examination by a trained medical technologist [
17]. In this study, the total time required for 20 leukopenic samples was approximately 115 min, and the time required for microscopic examination was about 61 min. However, the hands-on time of the Hematoflow method was only 15 min (less than 1 min per case). Therefore, the throughput of the Hematoflow method makes its use desirable especially in large hospitals with many severely leukopenic patients. Automated hematology analysis methods yielded good results with neutrophil and lymphocyte counts but less accurate results with monocyte counts. The 2 automated instruments used in this study did not perform differential counts in some severely leukopenic samples, and the correlation between the monocyte counts that they performed was poor. However, the reproducibility of Hematoflow counts was good in all 3 leukocyte subpopulations. When the WBC count was low, the number of countable WBCs on the slides also decreased and the reproducibility of manual counts tended to decrease for all cell populations (
Table 1). The reason for this may be that an insufficient number of WBCs are counted and that cells located in inappropriate sections of the hemacytometer are also counted in order to obtain a result from a leukopenic sample. Conversely, Hematoflow counts are more reliable as this method involves the analysis of a much higher number of cells, typically 10,000 events.
The results of the Hematoflow counts showed good correlation with those obtained both by the XE-2100 and by the DxH 800 instruments, except for monocytes, confirming that the use of the Hematoflow counting method in severely leukopenic samples is at least as reliable as that of conventional automatic analyzers. Furthermore, the XE-2100 instrument failed to report the differential count in 15% of the samples included in this study, whereas the DxH800 instrument and the Hematoflow method reported the counts in all samples.
The correlation between Hematoflow counts and manual counts was good enough to quantify neutrophils, lymphocytes and monocytes (r>0.8). Even in samples with WBC counts less than 100/µL, Hematoflow counts correlated well with manual counts (r>0.9), better than with other methods. This suggests that the Hematoflow could be the best method to monitor ANC and monocyte counts accurately in severely leukopenic patients. The correlation between the results for neutrophil and monocyte counts obtained by Hematoflow and each automatic analyzer was similar to the correlation between results obtained by the 2 automatic analyzers.
Hematoflow blast counts showed a remarkably good correlation with manual counts (r>0.8). None of the 154 cases showing less than 4% blasts by the Hematoflow method revealed any blasts in the manual counts. Eight of 21 cases (38.1%) showing more than 4% blasts with the Hematoflow method had blasts with the manual count. Cases with 1 to 3% blasts were not included in this study and the lowest percentage of blasts by manual count was 4%. However, as manual blast counts were very poorly reproducible and therefore could not serve as a reference to validate results obtained by the Hematoflow method, we could not determine the detection limit for blasts by the Hematoflow method in severely leukopenic samples. For example, in a sample with a WBC count of 500/µL, it may be impossible to manually count 100 cells. If only 25 cells were counted, and 1 blast and 24 other cells were found during examination, the percentage of blasts would be 4%. Therefore, the manual blast count in such severely leukopenic samples is only suitable to determine the presence or absence of blasts.
High sensitivity in the detection of residual blasts in leukemia patients is very important [
18,
19]. Although the Hematoflow method could differentiate between regenerating myeloblasts and lymphoblasts, this method cannot distinguish between normal regenerating myeloblasts and leukemic myeloblasts. Therefore, we do not recommend the Hematoflow method to monitor minimal residual disease in acute leukemia.
There were several false-positive detections of blasts by the Hematoflow method, with 13 cases (62%) showing more than 4% blasts while no blasts were identified on the smears. This is believed to be mainly due to contaminating debris in the gates for nucleated cells and incomplete separation of basophils from blasts.
Our results suggest that a differential count protocol that uses the Hematoflow method with the blast cutoff value set to 4% and reserves manual review for severely leukopenic samples only will help to reduce labor requirement in general laboratories that receive many severely leukopenic samples. Additionally, it will provide additional information on the character of the blasts [
20]. Since the separation of myeloblasts and basophils was not clear with the use of the Hematoflow method in certain cases, we recommend that blood smears should be manually reviewed in case of significant basophilia as well.
Another benefit of the Hematoflow method is that it provides information on lymphocyte subsets as well. Hematoflow reports 4 subsets of lymphocytes, including B-lymphocytes, CD16- T-lymphocytes, CD16+ T-lymphocytes, and T & NK lymphocytes. The clinical significance of discriminating monoclonal lymphoproliferative diseases including lymphoma and leukemia from reactive lymphocytosis has been well-established [
21,
22]. Although these subset data are not sufficient for the screening of lymphoid malignancies, they could provide valuable information for the diagnosis of certain patient subsets.
In conclusion, the Hematoflow method is useful for WBC differential counts in severely leukopenic samples. The Hematoflow method is a less labor intensive, convenient, and reproducible WBC differential counting method for the analysis of severely leukopenic samples. The correlation with manual counts is good in terms of neutrophil, lymphocyte, and monocyte counts. The Hematoflow method also provides information on blasts, and manual slide review is recommended when more than 4% blasts are found. However, further studies are necessary to establish the cutoff value for blasts that can be accurately reported by the Hematoflow method.
Go to :






 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download