Abstract
Nodular lymphoid hyperplasia of the stomach is a rare lymphoproliferative disorder. Here, we report a 38-year-old man who presented with multiple submucosal tumors of the stomach. Histologically, the lesions were characterized by multiple discrete submucosal nodules of lymphoid cells. The infiltrates between the lymphoid follicles were composed mainly of medium-sized lymphoid cells with abundant clear cytoplasm, as well as a few large cells with vesicular nuclei. The gastric mucosa exhibited multifocal lymphoid aggregates and some of the epithelial cells were infiltrated by small lymphocytes mimicking lymphoepithelial lesions. Histopathology was consistent with mucosa-associated lymphoid tissue lymphoma. However, the infiltrating lymphoid cells were positive for CD2, CD3, CD5, and CD7. In addition, polymerase chain reaction analysis of the immunoglobulin heavy chain and T-cell receptor gene rearrangements demonstrated polyclonality. This case was diagnosed as reactive lymphoid hyperplasia of the stomach.
Most previous cases of gastric pseudolymphomas were malignant lymphomas, specifically, extranodal marginal zone B-cell lymphomas [1]. The remaining cases were diagnosed as reactive lymphoid hyperplasia. Lymphoid hyperplasia of the gastrointestinal tract is clinically subdivided into focal (localized) and diffuse hyperplasia or focal and nodular lymphoid hyperplasia [2, 3]. In localized lymphoid hyperplasia of the large intestine, endoscopic lesions are either submucosal tumors or polyps [4]. Diffuse lymphoid hyperplasia is common and benign; it is thought to be a general response of mucosal lymphoid aggregates in the small and large intestine to an unknown stimulus [2]. Nodular lymphoid hyperplasia is characterized by multiple discrete mucosal nodules; however, gastric involvement is rare [3]. Moreover, there have not been any reported cases in the English literature of gastric nodular lymphoid hyperplasia presenting with multiple submucosal tumors or lymphomatous polyposis. Here, we present the histopathology, immunophenotype, and genotype findings of diffuse and nodular (polypoid) lymphoid hyperplasia of the stomach mimicking a polypoid type of mucosa-associated lymphoid tissue (MALT) lymphoma.
A 38-year-old man was referred to our hospital for multiple gastric submucosal lesions after an annual medical checkup. The patient did not have a history of congenital or acquired immunodeficiency or symptoms of abdominal pain, weight loss, or fever. He was immunocompetent, as shown by a complete blood count with differential and the absence of serum viral markers, such as anti-human immunodeficiency virus (HIV) antibodies. Esophagogastroduodenoscopy revealed multiple protruding lesions covered with normal mucosa on the body and antrum of the stomach (Fig. 1A). The lesions appeared as well-demarcated oval masses that varied in size from several millimeters to 1 cm. In addition, they were hypoechoic and originated in the submucosal layer without invasion into the deeper layers, as shown by endoscopic ultrasonography (Fig. 1B). The Campylobacter-like organism (CLO) test was negative. No pathologic lesions were found by colonoscopy. Computed tomography also did not reveal any abnormal thickening of the gastric wall or regional lymphadenopathy. Three mass lesions were resected by endoscopic submucosal dissection and endoscopic mucosal resection.
The resected polyps revealed several well-defined submucosal nodules of dense lymphoid infiltrates mimicking ectopic lymph nodes (Fig. 2A). These infiltrates had a diffuse and nodular architecture with primary and secondary lymphoid follicles (Fig. 2B). A few lymphoid follicles also had a prominent mantle zone and small germinal center (Fig. 2C). The lymphoid cells in the diffuse areas and primary follicles were composed predominantly of small cells, although there were a few multinucleated giant cells, which were considered to be Warthin-Finkeldey-type cells (Fig. 2D). The infiltrate between the follicles was composed mainly of medium-sized lymphoid cells with abundant clear cytoplasm and indented or round nuclei with small nucleoli, admixed with a small number of plasma cells. In addition, a few large cells with vesicular nuclei and one or two prominent nucleoli were identified (Fig. 2E). The gastric mucosa revealed multifocal lymphoid aggregates and some of the epithelial cells were infiltrated by small lymphocytes mimicking lymphoepithelial lesions (Fig. 2F).
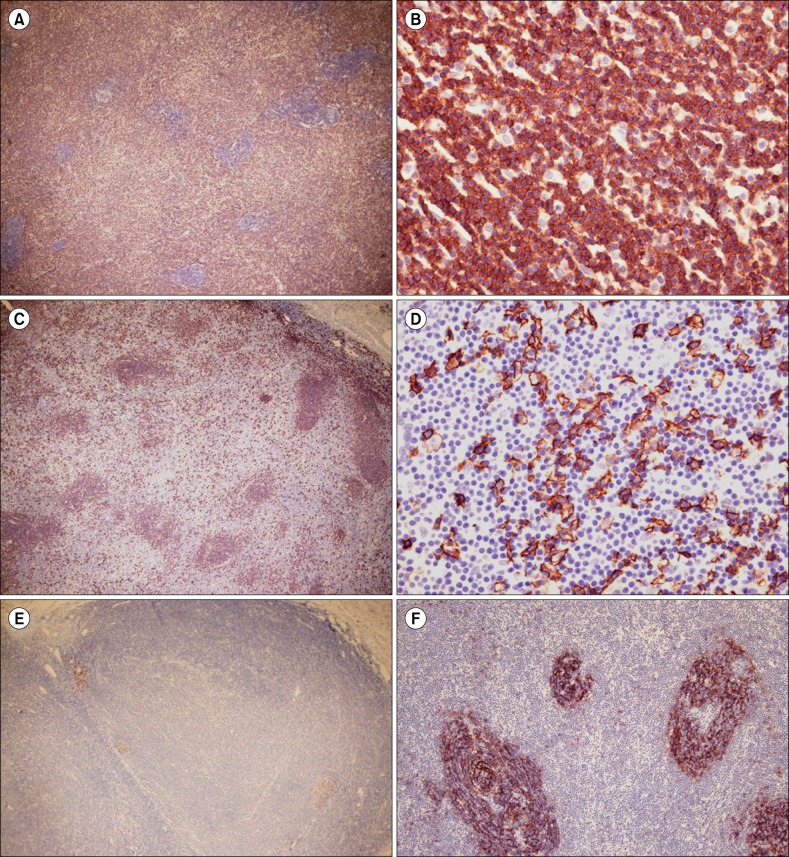
Immunohistochemistry tests showed that the lymphoid infiltrates, including the medium-sized cells, in the interfollicular areas expressed CD2, CD3, CD4, CD5, CD8, and CD43, but the small cells in the lymphoid follicles and the large cells in the interfollicular areas did not express these markers (Fig. 3A, 3B). However, the latter cell types expressed the CD20 antigen (Fig. 3C, 3D). CD10 and bcl-6-positive B lymphoid cells were confined to small germinal centers (Fig. 3E), but were negative for bcl-2. In addition, staining with CD21 and CD23 showed a normal reactive pattern in lymphoid follicles (Fig. 3F) and expanded follicular dendritic cell networks. CD30-positive large cells were rare. The plasma cells were polyclonal, as shown by kappa and lambda light chain immunohistochemistry.
Results of in situ hybridization for Epstein-Barr virus-encoded RNA were negative. In addition, no evidence of a chromosomal translocation was found by using interphase fluorescence in situ hybridization analysis of paraffin sections using the API2/MALT1 dual-color, dual-fusion translocation probe, which revealed two orange and two green signals. However, polymerase chain reaction analysis of the immunoglobulin heavy chain and T-cell receptor gene rearrangements using the standard Biomed-2 PCR protocol, demonstrated a polyclonal pattern on Gene Scanning. The patient was followed for 1 year and the polypoid lesions remained unchanged without any additional treatment.
This case is an example of nodular lymphoid hyperplasia with many submucosal nodules and polyps throughout the stomach. The differential diagnosis of gastric lymphoid lesions includes reactive processes and malignant lymphoma. In such cases, it is important to rule out extranodal marginal zone lymphoma. Here, the infiltrating lymphoid cells in the interfollicular area were similar to monocytoid B cells, because they had abundant clear cytoplasm. In addition, the lymphoid cells infiltrated the epithelial cells in the gastric mucosa, thus mimicking lymphoepithelial lesions. On the basis of endoscopy and histology findings, the initial diagnosis of this case was polypoid MALT-type lymphoma. However, monocytoid cells were reactive for CD3, but not for CD20, and large transformed B cells were negative for CD43 and bcl-2. Furthermore, the finding of diffuse lymphoid infiltrate in the mucosa and submucosa that lacks cellular atypia and exhibits immunoreactivity for B- and T-cell markers supported the diagnosis of a reactive process rather than malignancy.
To confirm this diagnosis, molecular genetic studies were performed. t(11;18)(q21;q21) is the most common chromosomal translocation associated with gastric MALT lymphoma and occurs in 15-31% of all cases [5-8]. Monoclonal immunoglobulin H (IgH) gene rearrangement has also been reported in 69-92% of all cases [6, 8, 9]. Although there is no consensus on the importance of molecular genetic studies, the results of these tests in this patient supported the diagnosis of reactive nodular lymphoid hyperplasia.
This case of nodular lymphoid hyperplasia differs from previously reported cases in several ways. For example, there is often a strong correlation between lymphoid tissue hyperplasia and Helicobacter pylori infection [10, 11]. The stomach is an unusual site for nodular lymphoid hyperplasia, especially when lymphoid infiltrates are concentrated in the mucosa. However, in this case, lymphoid infiltrates were present as well-defined submucosal nodules and H. pylori bacteria were not identified with either hematoxylin and eosin or Warthin-Starry staining. In focal lymphoid hyperplasia, lesions may be a chronic peptic ulcer or a segment of the gastric wall with thickening and cobblestoning of the mucosa [3]. In addition, diffuse nodular lymphoid hyperplasia of the small intestine or colon is usually associated with immune deficiency or malignant lymphoma [12]. Furthermore, follicular involution and Warthin-Finkeldey-type giant cells are usually observed in HIV-related benign lymphadenopathy. However, this patient did not have a history of immune deficiency or evidence of an underlying malignant lymphoma.
Histologically, localized lymphoid hyperplasia of the large intestine is usually characterized by large lymphoid follicles with active germinal centers as well as narrow surrounding mantle and marginal zones [4]. In contrast, in this case, the lymphoid follicles had small germinal centers and a prominent mantle zone. In addition, most of the hyperplastic monocytoid cells expressed CD3 and CD5 antigens. Most of the follicular dendritic cell meshwork showed a normal reactive pattern. This finding is common to nodular lymphoid hyperplasia of the colon. However, the significance of the expanded follicular dendritic cell meshwork is unknown. MALT lymphomas develop during prolonged reactive lymphoid proliferation, such as a response to chronic H. pylori gastritis.
Nodular lymphoid hyperplasia may be related to immune stimulation of the gut lymphoid tissue during bacterial infection [13]. Notably, lymphoid hyperplasia in the gastroduodenum is strongly associated with H. pylori infection, but regresses after H. pylori eradication [12, 14]. In patients with refractory H. pylori infection, the nodular lesions persist or the disease progresses [14]. Nodular lymphoid hyperplasia of the small intestine is associated with an increased risk of malignancy [15]. The prognosis of gastric nodular lymphoid hyperplasia without H. pylori infection is not known. More cases will need to be identified and followed up for an extended period to determine whether the hyperplasia remains benign or becomes malignant. This case has been followed for a relatively short time; however, long-term follow-up is planned.
In summary, we present a case of nodular (polypoid) lymphoid hyperplasia of the stomach that mimicked polypoid MALT lymphoma on endoscopic and pathologic examination. This case highlights the need for caution in over-diagnosing or overtreating this type of lesion.
References
1. Abbondanzo SL, Sobin LH. Gastric "pseudolymphoma": a retrospective morphologic and immunophenotypic study of 97 cases. Cancer. 1997; 79:1656–1663. PMID: 9128979.
2. Banks PM. Gastrointestinal lymphoproliferative disorders. Histopathology. 2007; 50:42–54. PMID: 17204020.

3. Ranchod M, Lewin KJ, Dorfman RF. Lymphoid hyperplasia of the gastrointestinal tract. A study of 26 cases and review of the literature. Am J Surg Pathol. 1978; 2:383–400. PMID: 736212.
4. Kojima M, Nakamura N, Itoh H, et al. Histological variety of localized lymphoid hyperplasia of the large intestine: histopathological, immunohistochemical and genotypic findings of 16 cases. J Clin Exp Hematop. 2009; 49:15–21. PMID: 19474513.

5. Remstein ED, Dogan A, Einerson RR, et al. The incidence and anatomic site specificity of chromosomal translocations in primary extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma) in North America. Am J Surg Pathol. 2006; 30:1546–1553. PMID: 17122510.

6. Wundisch T, Thiede C, Morgner A, et al. Long-term follow-up of gastric MALT lymphoma after Helicobacter pylori eradication. J Clin Oncol. 2005; 23:8018–8024. PMID: 16204012.
7. Nakamura S, Ye H, Bacon CM, et al. Clinical impact of genetic aberrations in gastric MALT lymphoma: a comprehensive analysis using interphase fluorescence in situ hybridisation. Gut. 2007; 56:1358–1363. PMID: 17525089.

8. Wang G, Auerbach A, Wei M, et al. t(11;18)(q21;q21) in extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue in stomach: a study of 48 cases. Mod Pathol. 2009; 22:79–86. PMID: 18820661.

9. Hummel M, Oeschger S, Barth TF, et al. Wotherspoon criteria combined with B cell clonality analysis by advanced polymerase chain reaction technology discriminates covert gastric marginal zone lymphoma from chronic gastritis. Gut. 2006; 55:782–787. PMID: 16423889.

10. Genta RM, Hamner HW, Graham DY. Gastric lymphoid follicles in Helicobacter pylori infection: frequency, distribution, and response to triple therapy. Hum Pathol. 1993; 24:577–583. PMID: 8505036.

11. Chen XY, Liu WZ, Shi Y, Zhang DZ, Xiao SD, Tytgat GN. Helicobacter pylori associated gastric diseases and lymphoid tissue hyperplasia in gastric antral mucosa. J Clin Pathol. 2002; 55:133–137. PMID: 11865009.

12. Tomita S, Kojima M, Imura J, et al. Diffuse nodular lymphoid hyperplasia of the large bowel without hypogammaglobulinemia or malabsorption syndrome: a case report and literature review. Int J Surg Pathol. 2002; 10:297–302. PMID: 12490983.
13. Khuroo MS, Khuroo NS, Khuroo MS. Diffuse duodenal nodular lymphoid hyperplasia: a large cohort of patients etiologically related to Helicobacter pylori infection. BMC Gastroenterol. 2011; 11:36. PMID: 21481240.

14. Rubio-Tapia A, Hernandez-Calleros J, Trinidad-Hernandez S, Uscanga L. Clinical characteristics of a group of adults with nodular lymphoid hyperplasia: a single center experience. World J Gastroenterol. 2006; 12:1945–1948. PMID: 16610004.

15. Ryan JC. Premalignant conditions of the small intestine. Semin Gastrointest Dis. 1996; 7:88–93. PMID: 8705262.
Fig. 1
(A) Endoscopy of the stomach showing multiple protruding mass-like lesions without mucosal change. (B) Endoscopic ultrasound imaging showing well-demarcated, oval, hypoechoic lesions of various sizes, originating in the submucosal layer without invading the deeper layers.

Fig. 2
(A) The resected polyps revealed two well-defined submucosal nodules of dense lymphoid infiltrates mimicking ectopic lymph nodes (original magnification ×2). (B) The lymphoid infiltrates had a diffuse and nodular architecture with primary or secondary lymphoid follicles (original magnification ×40). (C) A few lymphoid follicles had a prominent mantle zone and small germinal centers (original magnification ×100). (D) The lymphoid cells in the primary follicles were composed predominantly of small cells and a few multinucleated giant cells, which were considered to be follicular dendritic cells (original magnification ×400). (E) The infiltrate between the follicles was composed mainly of medium-sized lymphoid cells with abundant clear cytoplasm and indented or round nuclei with small nucleoli. A few large cells with vesicular nuclei and one or two prominent nucleoli were identified (original magnification ×400). (F) The gastric mucosa revealed multifocal lymphoid aggregates, and some of the epithelial cells were infiltrated by small lymphocytes mimicking lymphoepithelial lesions (original magnification ×400).
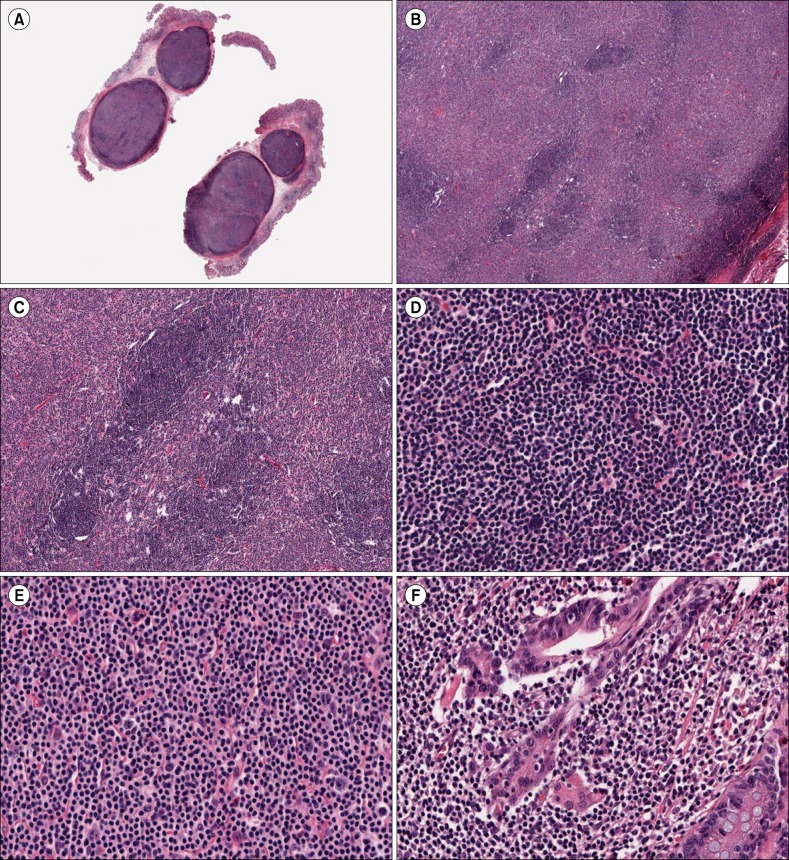
Fig. 3
(A) and (B) The lymphoid infiltrates, including the medium-sized cells in the interfollicular areas, were diffusely positive for CD3, but the small cells in the lymphoid follicles and large cells in the interfollicular areas were not (original magnifications ×40 (A) and ×400 (B)). (C) and (D) However, the latter cell types expressed CD20 (original magnifications ×40 (C) and ×400 (D)). (E) Bcl-6-positive B lymphoid cells were confined to small germinal centers (original magnification ×40). (F) Staining with CD21 showed a normal reactive pattern in the lymphoid follicle (original magnification ×100).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download