Hereditary hemolytic anemia (HHA) is a very rare disease entity characterized by premature red blood cell (RBC) destruction and anemia due to intrinsic RBC defects. The 3 main etiologies causing HHA, in order of frequency, are RBC membrane disorders, hemoglobin disorders, and RBC enzyme disorders. The prevalence of HHA in Korea is very low, because hereditary spherocytosis (HS) is less common in Asians than in Caucasians-with an incidence of 1 in 5000 births-and because Korea is not located in the thalassemia belt [1]. After the establishment of the HHA Working Party (WP) in the Korean Society of Hematology, 2 nationwide epidemiologic studies of HHA were conducted by the HHA WP: a 10-year retrospective survey carried out from 1997 to 2006 and a 5-year prospective survey carried out from 2007 to 2011 after the introduction of a standard operating procedure (SOP) for the diagnosis of HHA [2, 3].
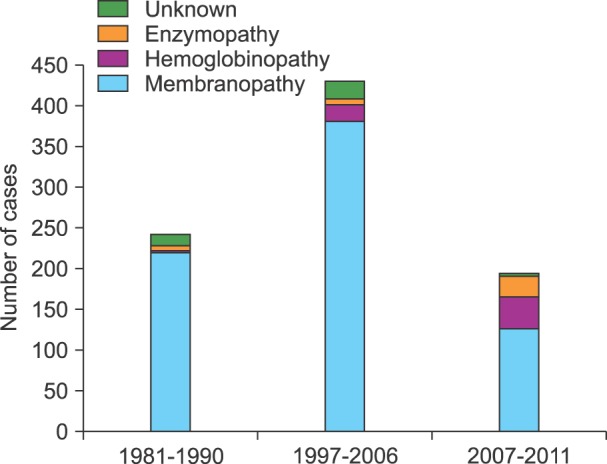
According to the results of a recent survey performed from 2007 to 2011 in Korea, 198 (122 men and 74 women) patients were diagnosed with HHA. The median age of the patients was 32 months (range: 0-187 months), and there were 127 (64.8%) patients with RBC membranopathies, 39 (19.9%) with hemoglobinopathies, 26 (13.3%) with RBC enzymopathies, and 3 (1.5%) patients had HHA of unknown etiology [3]. Data comparison between this study and studies performed during 1997-2006 and 1981-1990 [1-3] revealed that the proportion of hemoglobinopathy and enzymopathy has been gradually increasing (Fig. 1). This finding is probably due to an improvement in the diagnostic techniques for HHA, especially that of globin gene sequencing and RBC membrane protein and enzyme analysis, and an increase in multiracial marriages especially with South East Asians.
Nationwide epidemiologic studies have highlighted the need for an update of the SOP with newly developed, more accurate diagnostic methods for HHA, as well as the nationwide standardization of the methods and reporting of laboratory tests.
HS, the most common HHA characterized by abnormal RBC morphology and shortened RBC life span owing to extravascular hemolysis, is caused by a deficiency or dysfunction of membrane proteins associated with the RBC cytoskeleton: spectrin, ankyrin, band 3, protein 4.1, protein 4.2, and glycophorin. The genes encoding RBC membrane proteins and their respective chromosomal locations are known [4, 5].
The osmotic fragility (OF) test has been the most commonly used laboratory test for the screening of HS. However, the OF test has low sensitivity and specificity, and can give false negative results in 10-20% of HS cases, and false positive results in autoimmune hemolytic anemia. Furthermore, the OF test may be normal in cases of mild HS, in the presence of iron deficiency and obstructive jaundice, and in the recovery phase from an aplastic crisis [4]. In recently published guidelines for the diagnosis of HS, the eosin 5-maleimide (EMA) binding test and hypertonic cryohemolysis (HCH) test have been proposed as more reliable screening tests for HS. EMA interacts with the ε-NH2 group of Lys-430 on band 3 protein of the RBC membrane, and the EMA binding test uses flow cytometry to determine the amount of fluorescence derived from the EMA-bound band 3 protein. EMA-labeled HS RBCs with or without band 3 deficiency produce 25-30% lower mean channel fluorescence than normal RBCs. The HCH test measures % cryohemolysis at 540 nm after transfer of RBCs from 37℃ to 0℃ for 10 minutes. The EMA binding test and HCH test have a higher predictive value in the diagnosis of HS because there have been no reports of positive results in cases of immune or non-membrane-associated hemolytic anemia [4-6]. In addition, the flow cytometric OF (FC OF) test was recently developed as an assay that can replace the classical OF test [7]. The HHA WP compared the sensitivity, specificity, and predictive values of 3 screening tests (EMA binding test, FC OF test, and HCH test) in the diagnosis of HS. The results showed that both the EMA binding test and FC OF test had high sensitivity, specificity, and predictive values for the diagnosis of HS, whereas the HCH test had low sensitivity, specificity, and predictive values, and failed to discriminate between patients with HS and those with iron deficiency anemia.
For the confirmatory diagnosis of RBC membranopathy, RBC membrane protein analysis is usually performed by the sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) method [4, 5]. However, the SDS-PAGE method requires multiple step reactions and manual manipulations, which are labor-intensive and time consuming (about 1 week), and carry a greater risk of error owing to their complexity. Moreover, the SDS-PAGE method may be inaccurate in band 3 deficiency, because it yields results in the form of ratios of protein to band 3. The HHA WP is introducing quadruple-time of flight liquid chromatography-tandem mass spectrometry (LC-MS/MS) as a new method of RBC membrane protein analysis. LC-MS/MS is a very accurate method for measuring the mass of metabolites, drugs, proteins, lipids, and carbohydrates after ionizing the substrate. LC-MS/MS measures each protein separately, is not influenced by band 3 deficiency, and is known to be a simple, less time consuming (2-3 days), sensitive, and reproducible method for protein quantification.
Among the 20 or more RBC enzyme deficiencies known to cause HHA, deficiencies involving 3 enzymes such as glucose-6-phosphate dehydrogenase, pyruvate kinase, and pyrimidine 5'-nucleotidase are relatively common [8]. The Mayo Clinic measures 10 RBC enzymes to diagnose enzymopathy, including phosphofructokinase, triose phosphate isomerase, phosphoglycerate kinase, pyruvate kinase, glucose phosphate isomerase and hexokinase of the Embden-Meyerhof pathway; glucose-6-phosphate dehydrogenase of the hexose monophosphate shunt; adenylate kinase, pyrimidine 5'-nucleotidase and adenosine deaminase of the purine-pyrimidine pathway. For the confirmatory diagnosis of RBC enzymopathy, the RBC enzyme analysis method based on kinetic spectrophotometry has been used. Nevertheless, this method requires multiple step reactions and manual manipulations, which are labor intensive and time consuming, and carry a greater risk of error owing to their complexity. Therefore, the HHA WP is developing the multiplex RBC enzyme analysis using the ultra-performance liquid chromatography-tandem mass spectrometry method, which is expected to be a simple, rapid, sensitive, and reproducible quantification method for RBC enzymes [9].
Current HHA diagnostic guidelines recommend RBC membrane protein gene analysis when RBC protein analysis does not explain the clinical outcome and mode of inheritance in the patient with HS [4, 5]. Recently, the HHA WP started performing RBC membrane protein and enzyme gene analysis with the next generation sequencing method to define the common RBC membrane protein and enzyme gene abnormalities in Koreans. Thirteen genes encoding RBC membrane proteins (spectrin α and β, ankyrin, band 3, protein 4.1, protein 4.2, and glycophorins), and 15 genes encoding RBC enzymes (phosphofructokinase, triose phosphate isomerase, phosphoglycerate kinase, pyruvate kinase, glucose phosphate isomerase, hexokinase, phosphoglycerate kinase, acetyl cholinesterase, enolase, lactate dehydrogenase, glucose-6-phosphate dehydrogenase, adenylate kinase, pyrimidine 5'-nucleotidase, adenosine deaminase, glutathione reductase) will be the target of next generation sequencing. As the prevalence and etiologies of HHA are changing in Korea, nationwide epidemiologic studies will be carried out continuously with updated standardized SOP for HHA (Fig. 2).
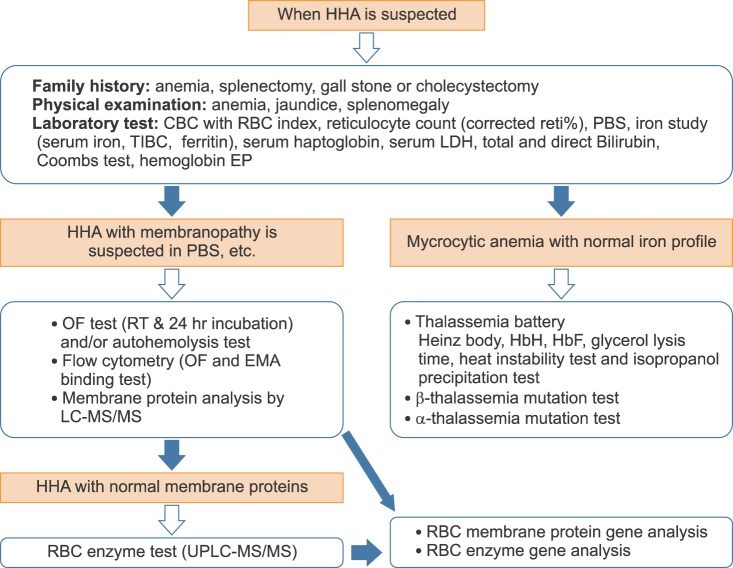 | Fig. 2Updated standard operating procedure for the diagnosis of hereditary hemolytic anemia. Abbreviations: HHA, hereditary hemolytic anemia; CBC, complete blood cell count; PBS, peripheral blood smear; TIBC, total iron binding capacity; LDH, lactate dehydrogenase; EP, electrophoresis; OF, osmotic fragility; RT, room temperature; EMA, eosin-5-maleimide; LC-MS/MS, liquid chromatography-tandem mass spectrometry; UPLC-MS/MS, ultra-performance liquid chromatography-tandem mass spectrometry. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download