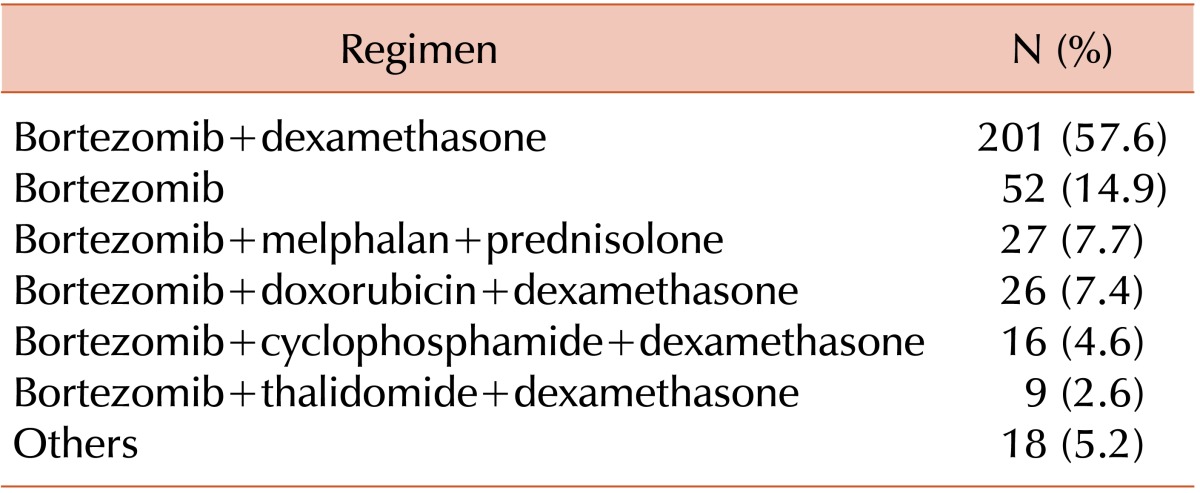
TO THE EDITOR: Despite the growing concern for the increased risk of infection associated with the use of new drugs for patients with myeloma, only few data have been reported until recently, especially for tuberculosis infections [1]. A recent analysis demonstrated the relationship between the use of bortezomib and the occurrence of tuberculosis in Korean patients with myeloma [2]. Investigating this relationship might be important in tuberculosis-prevalent areas such as Asian countries because it might influence physician decision-making with regard to the appropriate treatment regimen. Furthermore, previous study results showed a considerable incidence (7.0%) of tuberculosis during the course of treatment with a bortezomib-containing regimen. Considering the importance of this topic, we retrospectively analyzed our data to investigate the incidence of tuberculosis in patients treated with a bortezomib-containing regimen in our institute. Between October 2004 and August 2012, 285 patients with myeloma received 349 courses of bortezomib-containing regimens. The regimens administered are summarized in Table 1.
The median age of the patients included in our analysis was 60 years (range, 22-86 years). Of the patients, 50.9% were male. No occurrence of tuberculosis was encountered during the course of treatment with bortezomib-containing chemotherapy. However, we found 6 patients who developed tuberculosis during the follow-up period after myeloma diagnosis. Of the 6 tuberculosis cases, 4 were documented by acid-fast bacilli culture and 2 by tuberculosis pleurisy with increased adenosine deaminase levels. Of the 6 patients, 3 were previously exposed to bortezomib-containing treatment; the time interval from their last exposure to bortezomib to the tuberculosis diagnosis was from 5 to 21 months (5, 12, and 21 months, respectively). The other 3 patients developed tuberculosis during the course of their treatment with a thalidomide-containing regimen or alkylating agents. They were never exposed to bortezomib before developing tuberculosis. Thus, we found no case of tuberculosis during the course of treatment with a bortezomib-containing regimen in our study, except for the 3 patients who were previously exposed to bortezomib (3/285, 1.1%). The incidence rate of tuberculosis among our patients was lower than that in a recent study (7.0%). Although it cannot be directly compared with that of our study, the higher incidence of tuberculosis in the previous study might be associated with the other drugs combined with bortezomib. In our series, the most commonly combined drug was dexamethasone (57.6%), with 14.9% of the patients receiving bortezomib alone. Only 7.2% of the patients received bortezomib combined with cyclophosphamide or thalidomide. However, the study by Ahn et al. [2] showed that most patients received bortezomib in combination with thalidomide and cyclophosphamide. Our data imply that the addition of more combination drugs that may affect immune function, such as thalidomide or cyclophosphamide, might increase the patient's susceptibility to tuberculosis. To clarify this finding, a more extensive survey of the incidence of tuberculosis and the various myeloma treatment regimens is required. In conclusion, tuberculosis infection in the patients treated with a bortezomib-containing regimen was not common in our series. The increase in the susceptibility to tuberculosis by bortezomib-containing regimens might be more dependent on the other combination drugs.
References
1. Nucci M, Anaissie E. Infections in patients with multiple myeloma in the era of high-dose therapy and novel agents. Clin Infect Dis. 2009; 49:1211–1225. PMID: 19769539.

2. Ahn JS, Rew SY, Yang DH, et al. Poor prognostic significance of Mycobacterium tuberculosis infection during bortezomib-containing chemotherapy in patients with multiple myeloma. Blood Res. 2013; 48:35–39. PMID: 23589793.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download