TO THE EDITOR: The BK virus (BKV) is a human polyomavirus of the Papovaviridae family and causes clinical disease mainly in immunocompromised hosts such as patients infected with human immunodeficiency virus or transplant recipients [1]. Only a few cases of BKV encephalitis have been reported in hematopoietic stem cell (HSC) transplant recipients, with the majority of cases presenting with concurrent hemorrhagic cystitis [2-4]. In this paper, we report a patient with BKV encephalitis without concurrent hemorrhagic cystitis who received allogeneic stem cell transplantation from a matched sibling donor for treatment of a second relapse of diffuse large B cell lymphoma following autologous HSC transplantation (HSCT).
A 62-year-old woman with relapsed diffuse large B cell lymphoma (DLBCL) after autologous HSCT received an allogeneic peripheral blood stem cell transplantation from a sex-mismatched and ABO- and human leukocyte antigen-matched related donor after conditioning with fludarabine (30 mg/m2/day for 6 days) and intravenous busulfan (0.8 mg/kg every 6 hr for 2 days). She had been diagnosed with DLBCL 2 years previously, and treated with autologous HSCT as salvage therapy after the first relapse of the disease. Graft versus host disease (GVHD) prophylaxis consisted of 3 mg/kg/day of intravenous cyclosporine A (CsA) and intravenous methotrexate (15 mg/m2 on day 1 and 10 mg/m2 on days 3, 6, and 11). This therapy prevented development of acute GVHD. The serum level of CsA was adjusted to maintain a target trough serum level between 200 and 300 ng/mL. No hemorrhagic cystitis occurred during the course of immunosuppression.
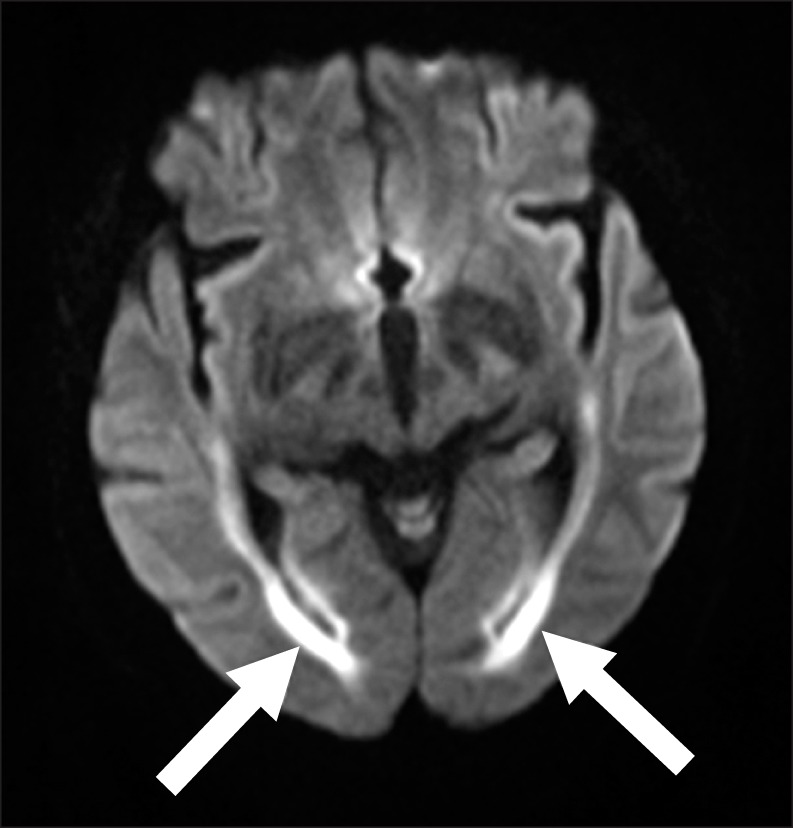
On post-transplant day 25, while the patient was still receiving CsA, memory loss, disorientation to time and place, and slurred speech were observed. Diffusion magnetic resonance imaging (MRI) showed no evidence of diffusion restriction, and only non-specific findings suggesting a subacute or chronic lacunar infarction at the right basal ganglia were found. The patient's disorientation recovered transiently over the next 24 hrs, although her neurological symptoms became progressively worse during the next 48 hrs. A repeat contrast-enhanced MRI performed 5 days after the onset of these symptoms showed no abnormal findings, with the exception of mild leukoaraiosis in the bilateral cerebral white matter, basal ganglia, and pons. A sleep and waking electroencephalogram (EEG) showed a slow, continuous generalized theta rhythm suggesting the presence of diffuse cerebral dysfunction. Based on this finding, CsA was switched to mycophenolate mofetil to exclude the possiblity of reversible posterior leukoencephalopathy syndrome, neurotoxicity caused by the immunosuppressive agent. Analysis of cerebrospinal fluid (CSF) showed normal glucose (71 mg/dL) and protein (19.5 mg/dL) levels and no evidence of pleocytosis. Microbiological examination of the CSF showed negative results for bacteria, mycobacteria, toxoplasma, herpes simplex virus type (HSV) I and II, cytomegalovirus (CMV), enterovirus, varicella-zoster virus, Epstein-Barr virus, and cryptococcus. Examination for BKV or John Cunningham (JC) viruses was not performed because there was no evidence of BKV encephalitis or progressive multifocal leukoencephalopathy on the MRI findings. The mental state of the patient deteriorated progressively with stupor without fever developing on post-transplant day 40. A follow-up sleep and waking EEG showed moderate to severe diffuse cerebral dysfunction, with evidence of progressive worsening of cerebral dysfunction. This finding led us to carry out a repeat MRI that showed the findings had changed since the initial scan. A diffusion-weighted image (Fig. 1) identified diffusion restriction areas in the post-frontal and parietal periventricular white matter, and along the precentral and postcentral subcortical white matter, suggesting new development of encephalitis.
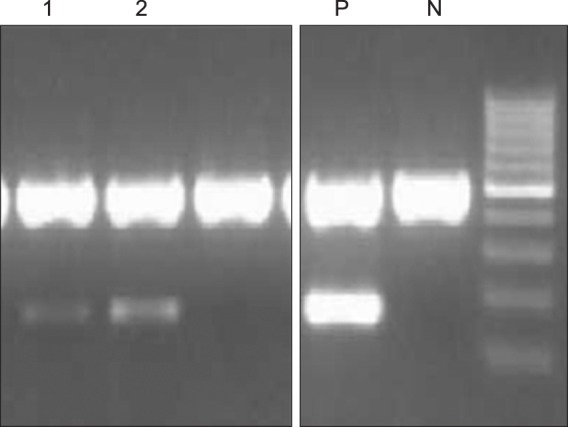
Because diffusion restriction is a common MRI finding in encephalitis cases, a follow-up CSF analysis including real-time PCR and PCR for BK virus was carried out on post-transplant day 48. Real-time PCR test and PCR were performed using a BKV quantitative real-time PCR kit (Bio-Core. Inc., Seoul, Korea) and a BKV PCR Kit (Bio-Core. Inc., Seoul, Korea), respectively, and showed positivity for the BK virus (Fig. 2). The viral load for BKV was 3,356 copies/mL, confirming the diagnosis of BKV encephalitis. PCR for BKV with a peripheral blood sample was also shown to be positive (Fig. 2).
PCR analysis of a urine specimen yielded negative results. PCR for other viruses such as HSV I, HSV II, enterovirus, varicella-zoster virus, Epstein-Barr virus, CMV, and JC were all negative. We did not discontinue immunosuppressive therapy when the diagnosis was established because we considered it too early to stop prophylaxis for GVHD. The patient died on post-transplant day 79 owing to probable aspiration pneumonia without recovery of mental stupor.
Primary BK virus infections occur during childhood with nearly 90% of children being infected by the age of 5 years [5]. Latent infection establishes in the renal epithelium and possibly other tissues including the brain. Chronic carrier status persists as long as the carrier remains immuno-competent, and reactivation may occur in immunodeficient patients [6-9]. The main clinical manifestations of BK virus infections are hemorrhagic cystitis, some types of encephalitis, and interstitial nephropathy in bone marrow transplant recipients, HIV-infected patients, and renal transplant recipients, respectively [7, 10, 11].
Several cases of BKV encephalitis have been reported in patients with immunocompromised status, although only 3 cases have been reported in HSC transplant recipients [2-4, 12-15]. Several points discriminate the present case from these previous reports. First, hemorrhagic cystitis was not observed in this case, unlike the previously reported clinical manifestations of BKV encephalitis in HSC transplant recipients, with 2 of the 3 cases developing hemorrhagic cystitis before the onset of symptoms associated with encephalitis [2, 4]. Second, the initial findings of MRI in our case were not compatible with encephalitis, despite the presence of severe neurological symptoms and signs. Although some pathogens causing viral encephalitis have a predilection for particular areas of the brain, no typical MRI characteristic for BKV encephalitis has been reported. One of common findings which have been reported for BKV encephalitis was diffusion restriction, known as a common finding of encephalitis [2, 3]. Three previously reported cases had MRI findings suggesting encephalopathy with the onset of neurological symptoms, but we were only able to find diffusion restriction in the third follow-up MRI, 19 days after the onset of symptoms. Third, reactivation of the BK virus occurred in a relatively short period (i.e., 23 days after the HSCT), whereas the previous cases showed late onset of neurologic symptoms, at least 100 days after HSCT. In addition to these atypical findings, several possible factors other than viral pathogens as causes encephalopathy, such as immunosuppressant medication (CsA) and uremia with blood urea nitrogen and creatinine levels being 62.2 mg/dL and 1.8 mg/dL at the onset of symptoms, respectively, made it difficult to accurately diagnose when the neurological symptoms appeared in our patient.
The definite diagnosis of BKV encephalitis in our patient was made by PCR of viral DNA in the CSF. This is the only established tool for diagnosing BKV encephalitis. The PCR result for BKV in a urine specimen was negative, and we were able to confirm that hemorrhagic cystitis did not develop concurrently in our case.
Because no effective treatment has been reported for the BK virus infection, early detection of the infection and reduction or discontinuation of immunosuppressant agents for restoration of immunity are known to be the best ways to control infection. This report emphasizes the importance of considering the presence of BKV encephalitis in allogeneic SCT recipients on immunosuppressant medications who present with neurological symptoms even though there is no sign of hemorrhagic cystitis.
References
2. Friedman DP, Flanders AE. MR Imaging of BK virus encephalitis. AJNR Am J Neuroradiol. 2006; 27:1016–1018. PMID: 16687535.
3. Lopes da Silva R, Ferreira I, Teixeira G, et al. BK virus encephalitis with thrombotic microangiopathy in an allogeneic hematopoietic stem cell transplant recipient. Transpl Infect Dis. 2011; 13:161–167. PMID: 21054716.

4. Behre G, Becker M, Christopeit M. BK virus encephalitis in an allogeneic hematopoietic stem cell recipient. Bone Marrow Transplant. 2008; 42:499. PMID: 18622417.

5. Gardner SD. Prevalence in England of antibody to human polyomavirus (B.k.). Br Med J. 1973; 1:77–78. PMID: 20791873.

6. Elsner C, Dorries K. Evidence of human polyomavirus BK and JC infection in normal brain tissue. Virology. 1992; 191:72–80. PMID: 1329338.

7. Reploeg MD, Storch GA, Clifford DB. Bk virus: a clinical review. Clin Infect Dis. 2001; 33:191–202. PMID: 11418879.

8. Shinohara T, Matsuda M, Cheng SH, Marshall J, Fujita M, Nagashima K. BK virus infection of the human urinary tract. J Med Virol. 1993; 41:301–305. PMID: 8106863.

9. Hogan TF, Borden EC, McBain JA, Padgett BL, Walker DL. Human polyomavirus infections with JC virus and BK virus in renal transplant patients. Ann Intern Med. 1980; 92:373–378. PMID: 6243896.

10. Nickeleit V, Hirsch HH, Binet IF, et al. Polyomavirus infection of renal allograft recipients: from latent infection to manifest disease. J Am Soc Nephrol. 1999; 10:1080–1089. PMID: 10232695.
11. Vago L, Cinque P, Sala E, et al. JCV-DNA and BKV-DNA in the CNS tissue and CSF of AIDS patients and normal subjects. Study of 41 cases and review of the literature. J Acquir Immune Defic Syndr Hum Retrovirol. 1996; 12:139–146. PMID: 8680884.

12. Behzad-Behbahani A, Klapper PE, Vallely PJ, Cleator GM. BK virus DNA in CSF of immunocompetent and immunocompromised patients. Arch Dis Child. 2003; 88:174–175. PMID: 12538331.

13. Voltz R, Jager G, Seelos K, Fuhry L, Hohlfeld R. BK virus encephalitis in an immunocompetent patient. Arch Neurol. 1996; 53:101–103. PMID: 8599551.

14. Vallbracht A, Lohler J, Gossmann J, et al. Disseminated BK type polyomavirus infection in an AIDS patient associated with central nervous system disease. Am J Pathol. 1993; 143:29–39. PMID: 8391217.
15. Bratt G, Hammarin AL, Grandien M, et al. BK virus as the cause of meningoencephalitis, retinitis and nephritis in a patient with AIDS. AIDS. 1999; 13:1071–1075. PMID: 10397537.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download