Abstract
Myelodysplastic syndrome (MDS) with eosinophilia is a rare condition and has yet to be classified under the 2008 World Health Organization classification. However, reports have described the prognostic significance of chronic persistent eosinophilia in MDS. Here, we report a case of a 67-year-old woman who was admitted to the hospital in July 2007 with generalized weakness, dizziness, and dyspnea on exertion persisting for 5 years. In the initial investigation, eosinophilia (22.1%) in peripheral blood and an increased proportion of eosinophils (5.6%) in normocellular bone marrow with dysplastic megakaryocytes and erythroid cells were noted. Eosinophilia was continuously detected during follow-up over 3 years. In a second bone marrow examination in August 2010, hypercellular bone marrow with similar features was observed. These findings led to the diagnosis of MDS with chronic persistent eosinophilia. To increase awareness of the prognostic significance of MDS with chronic eosinophilia, here we report a slow-progressing case of MDS with chronic persistent eosinophilia lasting over 6 years.
Myelodysplastic syndrome (MDS) is characterized by various forms of cytopenia with dysmorphic and ineffective hematopoiesis. This disease can affect patients of all ages but most commonly occurs in older patients with a mean age of 70 years [1, 2].
MDS has a tendency to transform into secondary AML and progress to bone marrow (BM) failure. Along with other hematologic malignancies, BM in MDS patients exhibits functional defects and contains myeloid cells with abnormalities in differentiation and maturation processes. Therefore, it is important to make the correct diagnosis and differentiate between hematological malignancies in patients showing similar BM characteristics [3].
To predict the prognosis of patients with MDS, the International Prognostic Scoring System (IPSS) is widely used. The IPSS is based on the percentage of BM blasts, cytopenia, and chromosomal abnormalities [4], but neither the IPSS nor the World Health Organization (WHO) takes into account the grade of eosinophilia. The prognostic significance of eosinophilia in MDS remains controversial [5].
To increase awareness of the prognostic significance of MDS with chronic eosinophilia, we report our experience of an indolent case of MDS with chronic eosinophilia persisting for 6 years.
A 67-year-old woman was admitted to our hospital in July 2007 with generalized weakness, dizziness, and dyspnea on exertion persisting for 5 years. Physical examination indicated a generally ill status. Abdominal examination revealed soft but distended abdomen, and no organomegaly was noted. Complete blood count (CBC) showed leukocyte count of 4.7×109/L, hemoglobin level of 6 g/dL, and platelet count of 140×109/L. Leukocyte differential count showed 39.3% segmented neutrophils, 22.1% eosinophils, 1% basophils, 29.3% lymphocytes, and 8.3% monocytes.
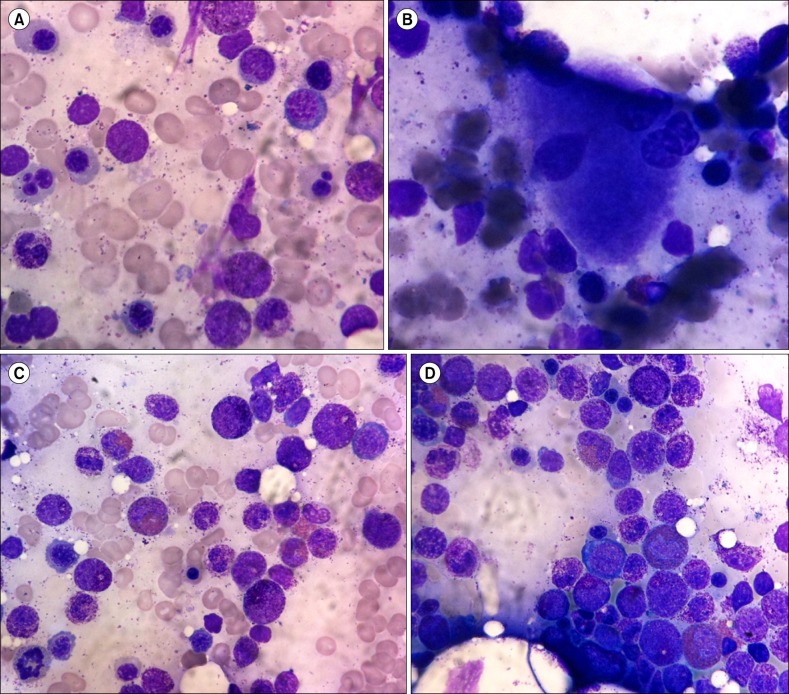
BM examination showed normocellular marrow with an adequate number of megakaryocytes but dysplastic changes to the megakaryocytes and erythroid series. Increased promyelocytes with granules (14.6%), eosinophils (5.6%), and basophils (7%) were observed (Fig. 1A, 1B). The patient's karyotype was normal (46 XX). Further molecular studies were recommended to the patient, but she refused, and therefore further evaluations were not carried out. The patient's initial diagnosis was MDS with eosinophilia and basophilia, which is subclassified as refractory cytopenia with multilineage dysplasia according to the 2008 WHO classification. After diagnosis, the patient was followed regularly. Her treatment did not include chemotherapies or BM transplantation; rather, she was conservatively managed by blood transfusion and medication to treat iron overload secondary to frequent transfusions. During the follow-up blood tests over 3 years, eosinophilia was continuously noted.
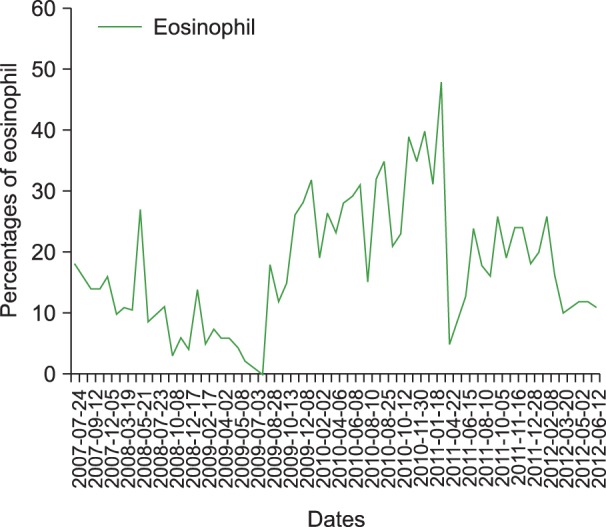
In August 2010, follow-up BM examination was performed. In the BM aspiration slide, hypercellular marrow with an adequate number of megakaryocytes and dysplastic changes in the erythroid series were noted. Promyelocytes with granules (11.9%) were still visible, and eosinophils (15.5%) with abnormal appearance were also detected (Fig. 1C, 1D). These findings led to the diagnosis of MDS with chronic persistent eosinophilia. A recent CBC in January 2012 still showed a high proportion of eosinophils (20%) (Fig. 2). The patient visits our hospital for regular follow-ups; she is generally in good condition but requires occasional blood transfusion for her anemia.
Currently, the percentage of marrow blasts, the degree of cytopenia, and cytogenetic patterns are well known prognostic indicators in MDS [5, 6]. However, several recent studies suggest a poor prognostic significance of chronic persistent eosinophilia in MDS. On the basis of the IPSS classification, MDS with eosinophilia is associated with a higher number of chromosomal abnormalities carrying intermediate or poor prognostic prediction for MDS, compared to MDS without eosinophilia [7]. The overall survival rate of patients with MDS and eosinophilia was also found to be lower than that of patients without eosinophilia. In addition, the evolution rate to AML was higher in MDS with eosinophilia than without eosinophilia, suggesting that eosinophilia is associated with a significantly reduced probability of AML-free survival. That study indicated that BM and peripheral blood eosinophilia in patients with MDS are predictors of poor prognosis [7].
In a recent study among 1,800 patients with MDS, Wimazal and colleagues [8] observed that patients presenting with eosinophilia had a significantly reduced survival compared with patients who did not have eosinophilia. Additionally, patients in the higher risk group (high risk and intermediate-2-risk group) had a prominent proportion of eosinophils and were more likely to experience AML evolution than those in the lower risk group (low-risk and intermediate-1-risk groups). On the basis of these findings, the authors concluded that the assessment of eosinophils in patients with MDS can be a useful prognostic marker for this malignancy.
However, even in patients with markedly increased numbers of eosinophils, the clinical course can be stable for years without any signs of rapid disease progression, such as an increase in blasts [9]. In a case report by Bakotic et al. [10], a 76-year-old male patient with MDS showing cytologically atypical BM and peripheral blood eosinophilia in association with ring chromosome 7, had favorable survival without disease progression with supportive care even though the patient presented with chromosomal abnormality. The clinical impact of chronic eosinophilia and the clinical relevance and prognostic effect of chromosomal abnormalities is not clear; therefore, whether eosinophilia in MDS is related to poor prognosis remains uncertain [9, 10].
The morphological differences between eosinophils and basophils in BM manifest in the promyelocyte stage; however, it is difficult to differentiate between these 2 cell types. Therefore, immature eosinophil promyelocytes may be misinterpreted as promyelocytes of basophils [11]. The similarities seen between the promyelocytes of eosinophils and basophils may have led to possible misinterpretations in our case. To differentiate eosinophilia and/or basophilia in MDS, further studies on the molecular and cytogenetic characteristics, such as by multicolor flow cytometry for FIP1L1/PDGFR, CBFβ/NYH11, and BCR/ABL, AML1/ETO can be performed [9]. However, because the patient refused further testing, we could not carry out any of these studies. Therefore, we do not have sufficient information to determine the coexistence of basophilia with eosinophilia. In addition, considering the presence of continuous eosinophilia during the patient's follow-up blood tests, diagnosis of MDS with persistent eosinophilia was more probable in this patient.
Our case demonstrated an indolent clinical course. Despite a number of reports that show eosinophilia as a poor prognostic factor in MDS, there are also indolent conditions of eosinophilia in MDS such as in the present case. The patient's survival over 6 years, even with a continuous eosinophilia proportion of over 10% during follow-up blood tests, demonstrates that not every case of chronic persistent eosinophilia is associated with poor outcome.
Overall, the exact correlation and clinical impact of eosinophilia and prognosis in MDS remains unclear [9, 10]. Further studies should be carried out on patients with MDS and chronic eosinophilia especially focusing on prognostic significance. The present case highlights the need for caution in interpreting the prognostic impact of eosinophilia in MDS as well as the need for inclusion of the prognostic significance of chronic eosinophilia in the current classifications of MDS.
References
1. Yang DW. Myelodysplastic syndrome. In : Park CJ, editor. Laboratory medicine. 4th ed. Seoul, Korea: E-public;2009. p. 185–189.
2. Bennett JM, Catovsky D, Daniel MT, et al. Proposals for the classification of the myelodysplastic syndromes. Br J Haematol. 1982; 51:189–199. PMID: 6952920.

3. Bennett JM, Komrokji RS. The myelodysplastic syndromes: diagnosis, molecular biology and risk assessment. Hematology. 2005; 10(Suppl 1):258–269. PMID: 16188686.

4. Greenberg P, Cox C, LeBeau MM, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997; 89:2079–2088. PMID: 9058730.

5. Verburgh E, Achten R, Louw VJ, et al. A new disease categorization of low-grade myelodysplastic syndromes based on the expression of cytopenia and dysplasia in one versus more than one lineage improves on the WHO classification. Leukemia. 2007; 21:668–677. PMID: 17301818.

6. Sanz GF, Sanz MA, Greenberg PL. Prognostic factors and scoring systems in myelodysplastic syndromes. Haematologica. 1998; 83:358–368. PMID: 9592987.
7. Matsushima T, Handa H, Yokohama A, et al. Prevalence and clinical characteristics of myelodysplastic syndrome with bone marrow eosinophilia or basophilia. Blood. 2003; 101:3386–3390. PMID: 12506028.

8. Wimazal F, Germing U, Kundi M, et al. Evaluation of the prognostic significance of eosinophilia and basophilia in a larger cohort of patients with myelodysplastic syndromes. Cancer. 2010; 116:2372–2381. PMID: 20209617.

9. Wimazal F, Baumgartner C, Sonneck K, et al. Mixed-lineage eosinophil/basophil crisis in MDS: a rare form of progression. Eur J Clin Invest. 2008; 38:447–455. PMID: 18445043.

10. Bakotic BW, Poniecka AW, Dominguez CJ, Benigno A, Donahue RP, Cabello-Inchausti B. Myelodysplastic syndrome with atypical eosinophilia in association with ring chromosome 7. A case report. Cancer Genet Cytogenet. 1999; 115:19–22. PMID: 10565294.
11. The Korean Society of Hematology. Hematology. 2nd ed. Seoul, Korea: E-public;2011. p. 304–315.
Fig. 1
Bone marrow (BM) aspirate smears showing dyserythropoiesis (A), dysmegakaryopoiesis (B), and increased promyelocytes with eosinophilic granules (C) in the initial BM study performed in July 2007. At a follow-up examination in August 2012, a BM aspirate smear showed increased immature eosinophils in the promyelocyte stage (D) (Wright stain, ×1,000).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download