Abstract
Background
A combination of the LISS/Coombs and enzyme methods is recommended for identifying unexpected antibodies. However, many laboratories in which tests are to be performed within the limits of medical fees covered by insurance, use only the LISS/Coombs method because the permissible medical fee is low as compared to the price of reagents required for both methods. The NaCl/Enzyme gel is used as a secondary assay when the LISS/Coombs gel test yields inconclusive results. We compared the frequency of unexpected antibody identified by LISS/Coombs gel with that obtained by the conditional combination of LISS/Coombs and NaCl/Enzyme gels. We aimed at establishing evidence-based guidelines for antibody testing.
Methods
From June 2007 to June 2012, antibody screening was performed for 69,986 samples; subsequently, antibodies were identified in samples showing positive screening results. These initial screenings and identifications were performed using the LISS/Coombs gel. We considered the results "inconclusive" when specific antibodies were not identified or reactions were too weak for accurate interpretation. For the inconclusive samples, we subsequently used NaCl/Enzyme gels.
Results
The overall detection rate of unexpected antibodies was 1.23%. Among the samples analyzed using NaCl/Enzyme gels, 40.2% showed results different from those obtained using LISS/Coombs gels. Moreover, 41.9% of samples with nonspecific reactions in LISS/Coombs gels showed clinically significant Rh or Kidd antibodies with NaCl/Enzyme gels.
All red cell antibodies other than naturally occurring anti-A and anti-B antibodies are defined as "unexpected antibodies." There are 2 types of unexpected antibodies: alloantibodies and autoantibodies. Production of alloantibodies may result from pregnancy, transfusion, transplantation, or injections of immunogenic material [1]. In Korea, alloantibodies have been reported in 0.3%-1.73% of patient samples depending on the study group, sensitivity, and test methods used [2-8].
The gel test is a commonly used column agglutination technique for unexpected antibody detection. Advantages of this test are that the readings are less prone to technical variation and more convenient to obtain than conventional tube methods, and multiple individuals can read the stable final reaction phase [9, 10]. To accurately identify unexpected antibodies, the use of a combination of the LISS/Coombs and NaCl/Enzyme gel tests is recommended. However, many laboratories in Korea perform antibody screening and identification tests using only the LISS/Coombs gel test because the coverage of related medical fees by insurance programs is too low as compared to the price of reagents required for both methods. Since June 2007, our laboratory has finalized the NaCl/Enzyme gel as a secondary test to be used only when the LISS/Coombs gel test result is inconclusive.
In this study, we compared the frequency of unexpected antibodies identified by the LISS/Coombs gel test only with the frequency of antibodies identified by the conditionally combined use of the LISS/Coombs and NaCl/Enzyme gel tests. We also analyzed the characteristics of the antibodies additionally identified by the NaCl/Enzyme gel test. Our results collectively provide evidence-based guidelines for unexpected antibody testing in Korea to maximize patient safety and cost effectiveness.
From June 2007 to June 2012, pretransfusion antibody screening tests were performed for 69,986 samples from transfusion candidates at Soonchunhyang University Hospital, Korea. We performed antibody identification tests on samples that showed positive screening test results. All initial screening and identification tests were carried out using the LISS/Coombs gel assay (DiaMed AG, Cressier sur Morat, Switzerland).
Briefly, a 50-µL sample of 0.8% screening or identification cell reagent (DiaMed AG) was added to the microtube of each gel card along with 25 µL of patient serum. After 15 min of incubation at 37℃, the card was centrifuged for 10 min at 910 rpm, and the agglutination reactions were examined macroscopically. An autocontrol was performed simultaneously by conducting a reaction between the patient's serum and 0.8% red blood cells, instead of reagent cells, from the same patient.
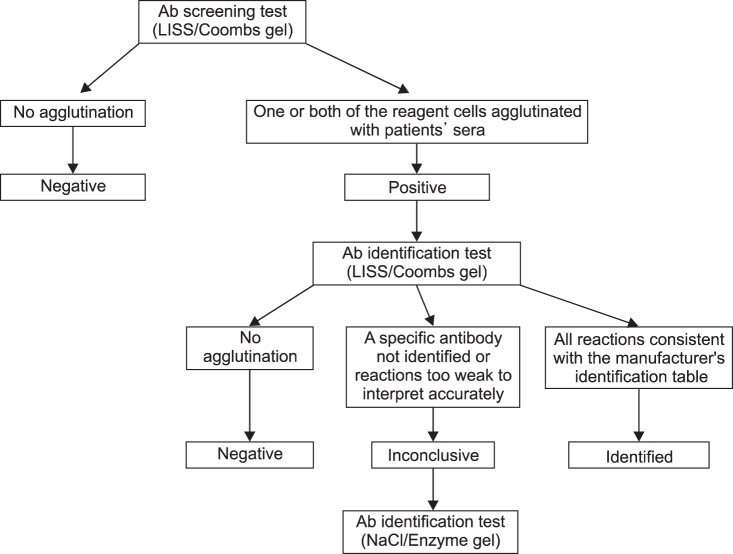
An antibody screening result was considered positive if one or both of the reagent cells agglutinated with the patient's serum in the LISS/Coombs gel test. An antibody was "identified" if all reactions in the 11 wells were consistent with the manufacturer's identification table and "negative" if no agglutination reaction occurred in any of the 11 wells [11]. Results were "inconclusive" when a specific antibody was not identified or the test reactions were too weak to interpret accurately. NaCl/Enzyme gel (DiaMed AG) was added for additional antibody identification if the results of the LISS/Coombs gel test were inconclusive (Fig. 1).
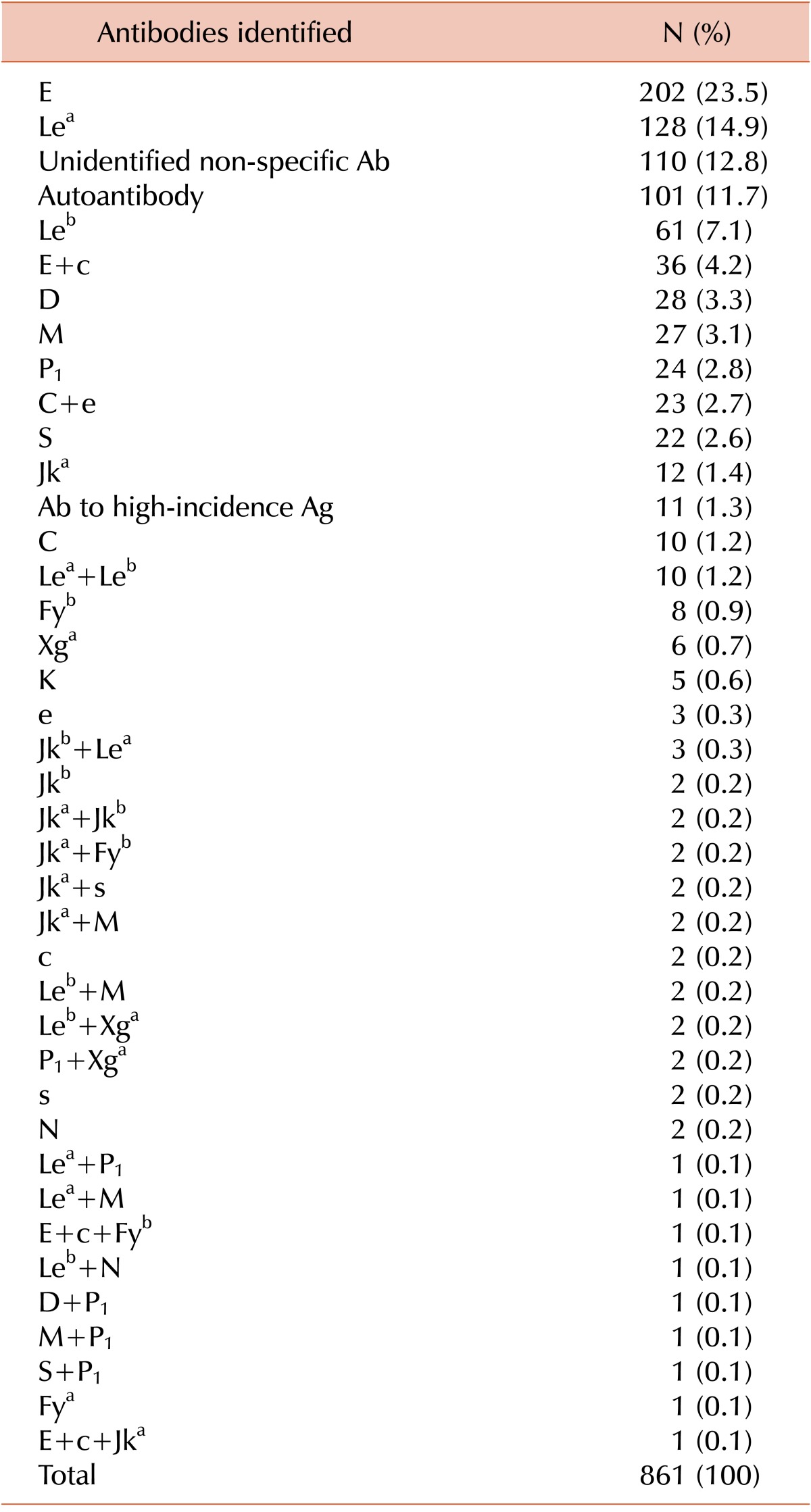
Among the 69,986 samples, 861 (1.23%) showed positive results in antibody screening tests. The final identification results of these samples are shown in Table 1. Briefly, anti-E was the most commonly identified antibody (202 of 861 samples, 20.3%), and anti-Lea was the second most-commonly identified (128 of 861 samples, 14.9%). Autoantibodies were present in 101 (11.7%) samples. The specificity of the antibodies could not be determined in 110 (12.8%) samples (Table 1).
Of the 861 positive samples, 516 (59.9%) were from female patients and 345 (40.1%) were from male patients. The age distribution was as follows: 0-10 yr, 10 (1.2%); 11-20 yr, 38 (4.4%); 21-30 yr, 56 (6.5%); 31-40 yr, 108 (12.5%); 41-50 yr, 145 (16.8%); 51-60 yr, 184 (21.4%); 61-70 yr, 168 (19.5%); 71-80 yr, 113 (13.1%); 81-90 yr, 35 (4.1%); and 90-100 yr, 4 (0.5%).
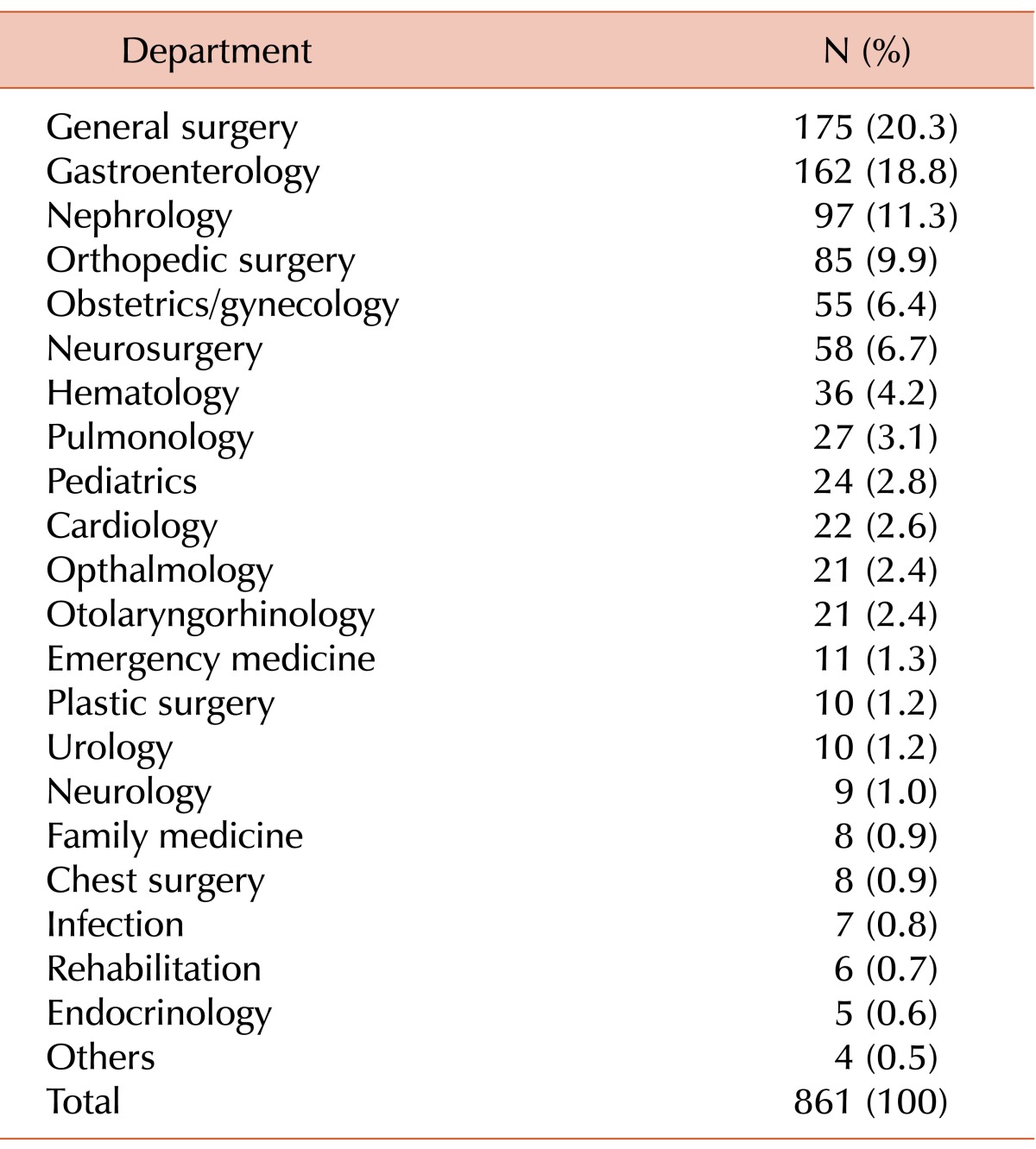
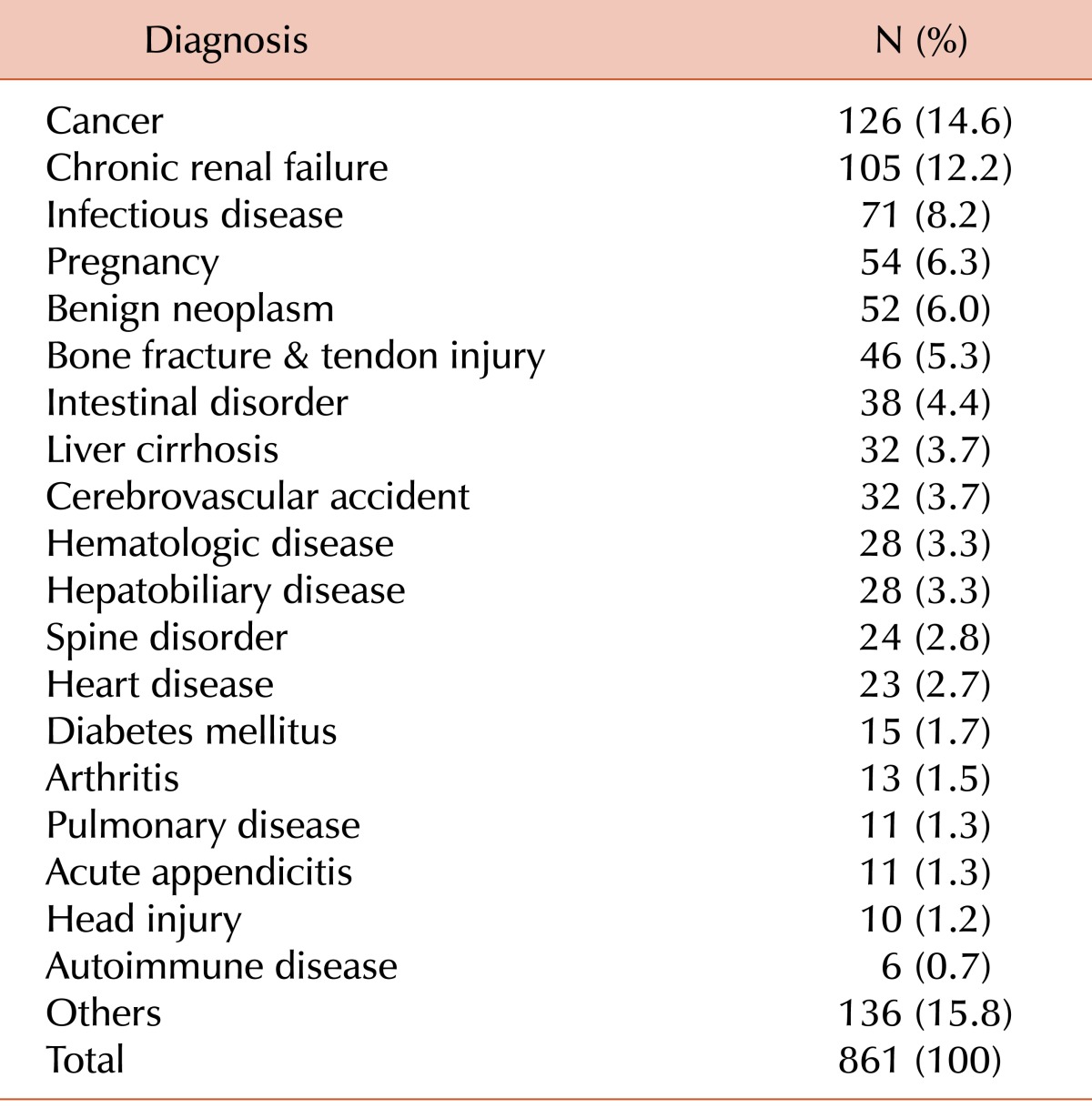
The distribution of patients with antibodies in their blood samples grouped according to clinical department and their diagnoses are shown in Tables 2 and 3, respectively. The highest numbers of patients were admitted to the general surgery department, followed by the gastroenterology department. Cancer and chronic renal failure were the most frequent diagnoses among patients with antibodies in their blood samples.
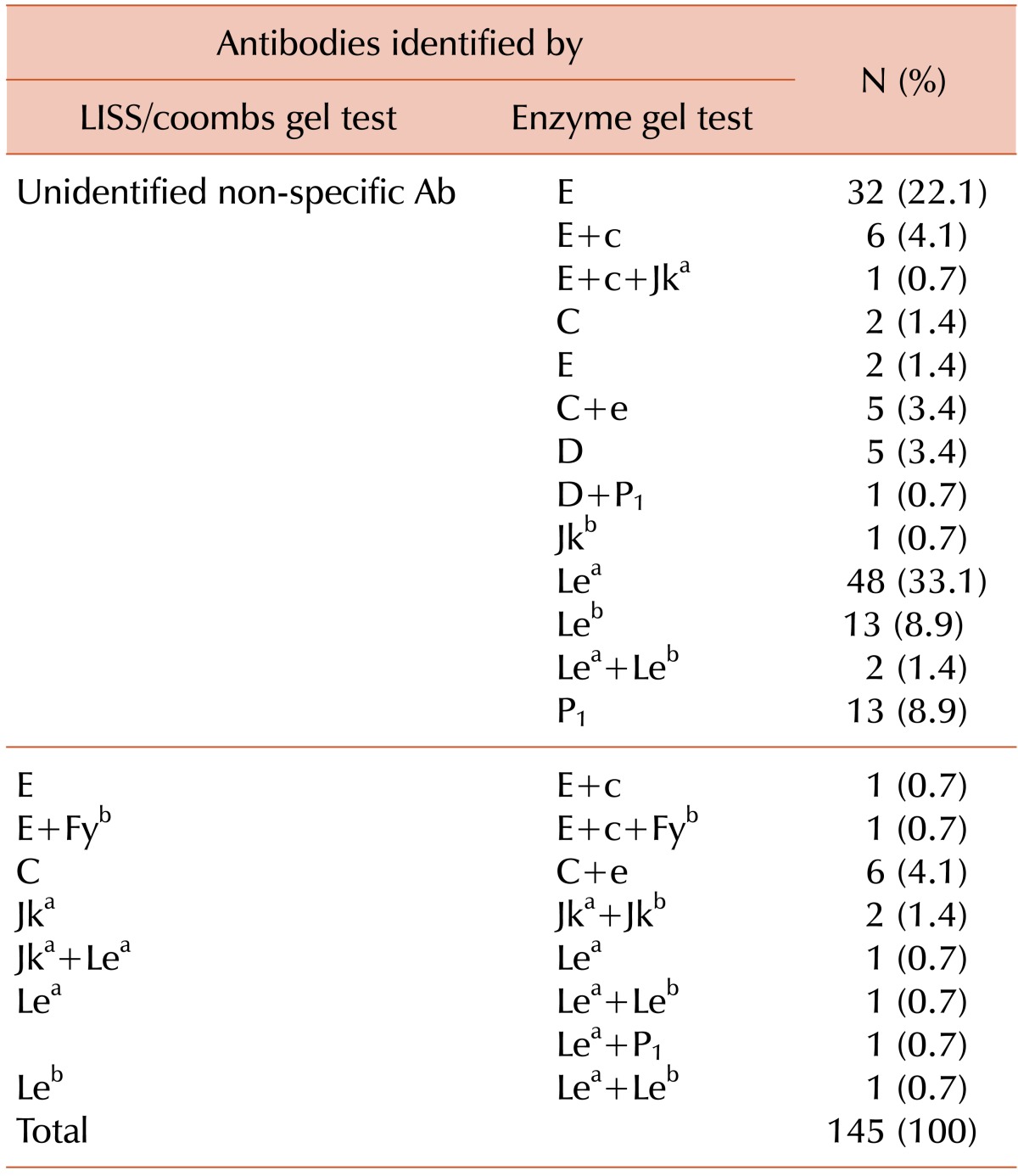
Of the 861 samples that were subjected to antibody identification with the LISS/Coombs gel test, 361 (41.9%) showed inconclusive results. The NaCl/Enzyme gel test was then performed on these samples, and 216 showed the same results as the LISS/Coombs test. Among the remaining 145 samples showing different results between the 2 tests, 131 samples that previously showed nonspecific reactions on the LISS/Coombs gel tests now showed specific antibodies on the NaCl/Enzyme gel test, and 55 (41.9%) of these samples showed clinically significant antibodies such as Rh and Kidd (Table 4). In the remaining 14 samples, specific antibodies were identified in the LISS/Coombs gel, but their reactivity was weak. The NaCl/Enzyme gel test showed strong reactivities for these samples, and additional antibodies were identified in all but 1 sample; in this last sample, Jka and Lea antibodies were detected by LISS/Coombs, but only the Lea antibody was detected on NaCl/Enzyme gel (Table 4).
Clinically significant, unexpected, red blood cell antibodies can cause hemolytic transfusion reactions, secondary to accelerated destruction of a significant proportion of transfused red blood cells. Therefore, screening for unexpected antibodies should be part of all pretransfusion testing, with subsequent antibody identification in the event of a positive screening result [11, 12]. The 2 principal techniques for unexpected antibody detection are the indirect antiglobulin and enzyme methods; combining the 2 methods is recommended for exact identification. Treating red blood cells with proteolytic enzymes enhances their reactivity with antibodies in the Rh, P, I, Kidd, and Lewis systems, but destroys or weakens reactivity with other antibodies, most notably those in the Duffy and MNS systems [13]. In the commonly used column agglutination technique, indirect antiglobulin and enzyme methods correspond to the LISS/Coombs and NaCl/Enzyme gel tests, respectively.
In laboratory management, cost-effectiveness should be considered for most tests. This is especially true in Korea, where health insurance is a compulsory social system that covers the whole population; therefore, laboratory tests must be performed within the limits of the medical fees covered. Owing to these economic limitations, we cannot always perform the full range of tests required for maximum accuracy. For example, the running costs of antibody screening, antibody identification by LISS/Coombs gel, and antibody identification by NaCl/Enzyme gel tests are KRW 1,857 ($1.7), KRW 9440 ($8.5), and KRW 8633 ($7.8), respectively. However, the medical fees provided by the National Health Insurance System are KRW 6220 ($5.6) for antibody screening and KRW 14,590 ($13.2) for antibody identification. Although performing the antibody identification test with both LISS/Coombs and NaCl/Enzyme gel tests is ideal, the permitted fee is calculated on the assumption that a single method (LISS/Coombs or NaCl/Enzyme) is performed. Performing antibody screening and identification tests with both methods, combined with the personnel expenses of laboratory doctors or technicians, would exceed the medical fees covered by insurance.
Some researchers, however, have argued that the clinical significance of antibodies that react only with enzyme techniques is questionable [13-15], and as such, routine use of enzyme techniques instead of the LISS/Coombs method is not recommended.
Based on the data collected from these tests in our hospital since 2007, we concluded that, to ensure both patient safety and cost effectiveness, the NaCl/Enzyme gel test should be used at least when the antibody identification test results by LISS/Coombs gel are inconclusive. The overall detection rate of unexpected antibodies in our study was 1.23%, which is similar to the results of previous studies in Korea [2-8]. Among the samples for which additional NaCl/Enzyme gel tests were performed, 40.2% showed results different from those of the LISS/Coombs test. Moreover, 41.9% of samples that showed nonspecific reactions in LISS/Coombs gel tests had clinically significant Rh or Kidd antibodies. Therefore, clinically significant antibodies that could cause hemolytic transfusion reactions could likely be missed with the use of only the LISS/Coombs gel test.
One of the possible limitations of our study is that we did not perform the antibody identification tests by LISS/Coombs and NaCl/Enzyme gels simultaneously on all samples with positive screening results. Therefore, further study using NaCl/Enzyme gel tests for samples showing obvious results with LISS/Coombs gel tests is required to determine how many additional significant antibodies can be detected by the NaCl/Enzyme gel tests. According to Lee et al. [16], the number of samples identified only by NaCl/Enzyme gels and showing negative results by LISS/Coombs gels were only 4 of 79 (5.1%). Additionally, in their study, the number of samples showing inconclusive results by LISS/Coombs gel tests was 36 of 79 (45.6%), a value similar to that found in our study.
In conclusion, considering both patient safety and cost effectiveness, we recommend the use of a conditional combination of LISS/Coombs gel and NaCl/Enzyme gel tests for unexpected antibody identification, especially in laboratories that need to perform the tests within a prespecified budget.
References
1. Roback JD, Grossman BJ, Harris T, Hillyer CD, editors. Technical manual. 17th ed. Bethesda, MD: American Association of Blood Banks;2011. p. 463.
2. Lee MH, Cho HI, Kim SI. A study on blood group antibodies in the Korean. Korean J Hematol. 1986; 21:243–256.
3. Park MH, Kim SH, Song WH, Cho HI. Screening of irregular red cell antibodies by microplate method in transfusion candidates. Korean J Clin Pathol. 1986; 6:473–481.
4. Han KS, Oh WI, Park MH, Kim EC, Kim SI. Irregular blood group antibodies in Korean. Korean J Hematol. 1989; 24:145–153.
5. Cho HI. Studies on preparation of red cell panel and red cell antibodies in Koreans. Korean J Clin Pathol. 1982; 2:105–112.
6. Kim BS, Kim HO, Song KS, Lee SY. Frequency of irregular antibodies detected by type and screen procedure. Korean J Blood Transfus. 1990; 1:47–49.
7. Kim HO, Won DI, Kwon OH. The frequencies of unexpected antibodies in transfusion candidates and selection of cross-matching method. Korean J Blood Transfus. 1993; 4:35–41.
8. Song DH, Moon IS, Hong SJ, Park JH, Kim JG, Jeon DS. Frequency and distribution of unexpected antibodies of Koreans. Korean J Blood Transfus. 1998; 9:191–200.
9. Knight RC, de Silva M. New technologies for red-cell serology. Blood Rev. 1996; 10:101–110. PMID: 8813342.

10. Brecher ME, editor. Technical manual. 15th ed. Bethesda, MD: American Association of Blood Banks;2005. p. 284–285.
11. Shin JH, Lee JY, Kim JH, Kim HR, Lee JN. Screening and identification of unexpected red cell antibodies by simultaneous LISS/Coombs and NaCl/Enzyme gel methods. J Korean Med Sci. 2009; 24:632–635. PMID: 19654944.

12. Chapman JF, Elliott C, Knowles SM, Milkins CE, Poole GD. Guidelines for compatibility procedures in blood transfusion laboratories. Transfus Med. 2004; 14:59–73. PMID: 15043595.

13. Roback JD, Grossman BJ, Harris T, Hillyer CD, editors. Technical manual. 17th ed. Bethesda, MD: American Association of Blood Banks;2011. p. 443.
14. Issitt PD, Combs MR, Bredehoeft SJ, et al. Lack of clinical significance of "enzyme-only" red cell alloantibodies. Transfusion. 1993; 33:284–293. PMID: 8480348.

15. Pereira A, Mazzara R. Enzyme techniques in pretransfusion testing. Transfusion. 1993; 33:884. PMID: 8236433.

16. Lee JS, Cho D, Shin MG, Ryang DW. Usefulness of NaCl/Enzyme gel test for the identification of unexpected antibodies. Korean J Lab Med. 2006; 26:204–209. PMID: 18156726.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download