This article has been
cited by other articles in ScienceCentral.
TO THE EDITOR: Heparin-induced thrombocytopenia (HIT) is a significant complication of heparin therapy. Risk factors for HIT include the type of heparin used, duration of heparin exposure, and clinical setting. Here, we report a 76-year-old man who presented with life-threatening pulmonary thromboembolism and deep-vein thrombosis, preceded by a dramatic decrease in platelet count 1 week after radical cystectomy for transitional cell bladder carcinoma. He was diagnosed with type II HIT caused by a small amount of unfractionated heparin mixed into his total parenteral nutrition (TPN) solution. One thousand units heparin per day were added to his TPN for 1 week. A heparin-platelet factor-4 antibody enzyme-linked immunosorbent assay of his serum was strongly positive. After cessation of TPN infusion, argatroban was started for thrombosis. His platelet count gradually increased to a normal level, and the thrombosis was treated successfully. This case suggests HIT should be suspected in patients with typical clinical manifestations and risk factors, even if the infused heparin dose is small.
Heparin is widely used in a variety of medical therapies from the treatment of life-threatening acute thromboembolisms to the maintenance of indwelling vascular catheter patency. However, heparin therapy is often complicated by thrombocytopenia. The incidence of heparin-induced thrombocytopenia (HIT) in patients who receive heparin including unfractionated heparin and low-molecular-weight heparin is 0.1-5% [
1]. There are 2 types of HIT. Type I HIT is a relatively common non-immune reaction that causes an asymptomatic, transient, and mild decrease in platelet count. In contrast, type II HIT is an immune complex disorder involving heparin bound to the platelet-specific chemokine, platelet factor-4 (PF4). These heparin-PF4 complexes activate both platelets and endothelial cells, resulting in platelet consumption and endothelial injury with thrombosis and disseminated intravascular coagulation [
2]. Heparin use could go unnoticed if it is not applied as a conventional anticoagulant but rather as a supportive agent to maintain vasc,ular patency. However, unrecognized HIT can lead to life-threatening complications. Therefore, it is important to suspect HIT in patients who develop acute thrombosis or thrombotic tendency preceded by a decrease in platelet count during or soon after heparin therapy, even if the infused amount is small. Immediate discontinuation of heparin administration and initiating alternative anticoagulant therapy are critical for avoiding further thrombosis, even before laboratory results are available [
3]. To the best of our knowledge, this is the first report on a patient who experienced type II HIT after low-dose heparin added to his total parenteral nutrition (TPN) for 1 week.
CASE
A 76-year-old man visited our hospital with gross hematuria and urinary hesitance for 2 months. A computed tomography (CT) scan showed a soft-tissue mass involving the bladder wall, which was defined as high-grade transitional cell carcinoma by cystoscopic biopsy. There was no evidence of lymph node or distant metastasis. He received radical cystectomy with an ileal conduit for T4N0M0 bladder cancer. Four days after the operation, his complete blood count showed mild leukocytosis and anemia, but his platelet count was 151×109/L, which was within the reference range (130-400×109/L). One week after the operation, hemorrhagic body fluid was noticed through the internal drainage tube inserted into the peritoneum. At this point, marked thrombocytopenia with a platelet count of 21×109/L was detected. His prothrombin time and activated partial thromboplastin time were both normal. A peripheral blood smear did not show any abnormal findings except the markedly decreased platelet count. Despite platelet transfusion, his platelet count did not increase.
To elucidate the cause of thrombocytopenia, all drugs prescribed to him since hospital admission were reviewed. His medication consisted of ceftizoxime, antitussive agents, histamine 2 receptor blockers, and TPN. Assuming the antibiotics induced thrombocytopenia, ceftizoxime was switched to moxifloxacin. However, severe thrombocytopenia (<20×10
9/L) was sustained for several days despite cephalosporin discontinuation. Ten days after the operation, the patient complained of sudden dyspnea and right-lower extremity pain with swelling. Arterial blood gas analysis revealed the following: pH 7.44; PaCO
2, 28.8 mmHg; PaO
2, 52.2 mmHg; and HCO
3-, 19.3 mmol/L. A chest radiograph showed no significant infiltration. D-dimer was greater than 109.52 nmol/L (normal level: <2.19 nmol/L). Under the suspicion of combined pulmonary thromboembolism (PTE) and deep-vein thrombosis (DVT), lower-extremity venous Doppler ultrasonography and chest CT scan were performed. The results revealed PTE in both lungs (
Fig. 1) and extensive segmental DVT from the right common femoral vein to the popliteal vein and distal calf vein (
Figs. 2 and
3). Since it was not possible to adopt heparin anticoagulation due to bloody exudates from the internal drainage tube, an infrarenal inferior vena cava filter was inserted through the right internal jugular vein.
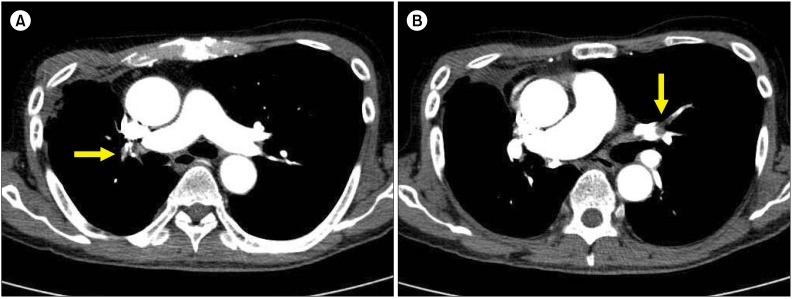 | Fig. 1Right pulmonary artery (yellow arrow) (A) and left pulmonary artery (yellow arrow) (B) occluded by PTEs. 
|
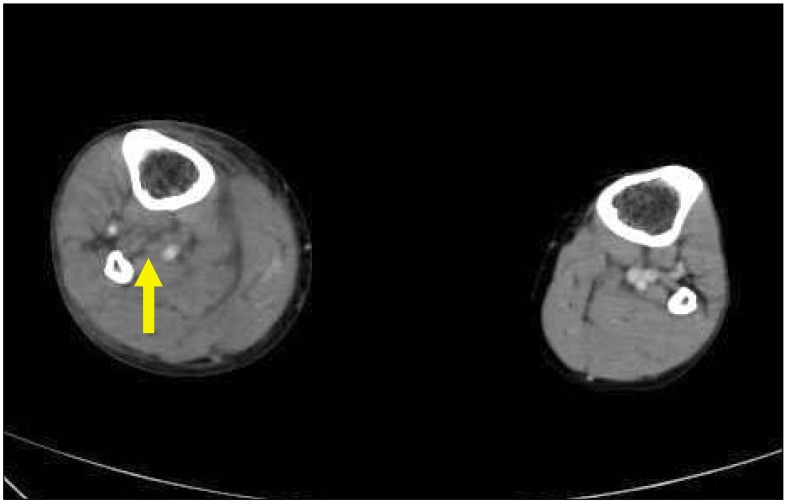 | Fig. 2Filling defect of right distal calf vein (yellow arrow) due to thrombosis and swollen right lower leg. 
|
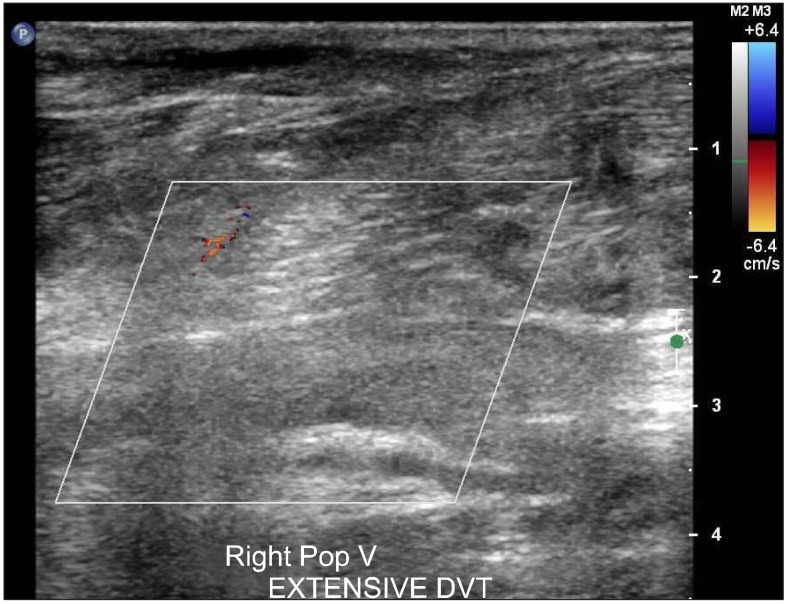 | Fig. 3Near total obstruction of right popliteal vein due to extensive DVT. 
|
Although the patient was not directly prescribed any form of heparin, we did not exclude the possibility of HIT, because his clinical manifestations matched the typical courses of HIT patients. Thirteen days after the operation, after completing a thorough review of medicines administered, we discovered that a small amount of unfractionated heparin was present in his TPN formula to maintain the patency of peripheral vascular catheters. One thousand units (1 mL) heparin daily, for a total of 7,000 units, were mixed into his TPN over 1 week. TPN was immediately stopped (i.e., 13 days after the operation), and serum PF4 antibodies were tested. Argatroban administration was initiated on the same day. The test results came out 2 days later: the heparin-PF4 antibody enzyme-linked immunosorbent assay (ELISA, PF4 Enhanced; Gen-Probe GTI Diagnostics, Inc., Waukesha, WI, USA) optical density (OD) value was 3.059 (cutoff: 0.4), indicating strong reactivity. Finally, the patient was diagnosed with type II HIT. After cessation of heparin-supplemented TPN and initiation of argatroban therapy, his platelet count started increasing, reaching the normal range in 6 days (
Fig. 4). The required amount of supplemental oxygen was reduced as dyspnea gradually improved, and the inferior vena cava filter was removed. After the platelet count and PTE stabilized, argatroban was switched to warfarin with some overlapping period. After 6 months of anticoagulation therapy, a CT scan revealed that both PTE and DVT had resolved completely. At present, the patient has no sequelae of HIT.
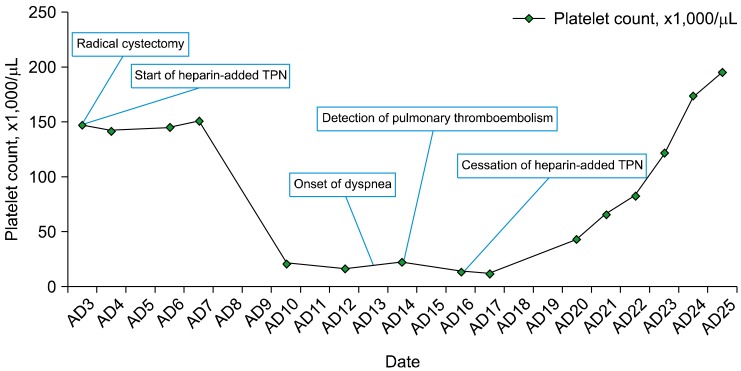 | Fig. 4Decrease and recovery of platelet count with respect to the start and cessation of heparin-supplemented TPN. Abbreviations: AD, admission date; TPN, total parenteral nutrition. 
|
Go to :

DISCUSSION
HIT involves 2 distinct clinical coagulopathic aspects: thrombocytopenia and vascular thrombosis [
4]. Heparin, a negatively charged glycosaminoglycan, binds to the positively charged protein PF4 and forms heparin-PF4 complexes [
2,
3]. These complexes bind to HIT IgG antibodies, thereby constructing large immune complexes that activate platelets through Fcγ-IIa receptors. This process causes the release of prothrombotic platelet-derived microparticles and more PF4 complexes in a positive-feedback loop [
1,
3,
5]. HIT can be difficult to diagnose, if the patient has multiple possible causes of thrombocytopenia. HIT should usually be suspected when there is unexplained thrombocytopenia with a platelet count <100-150×10
9/L or a 30-50% drop from baseline platelet count within 5-10 days after heparin exposure [
3]. HIT is diagnosed on the basis of both clinical and laboratory findings, i.e., the 4Ts score and antigen-detecting immunoassays [
3,
5-
7]. The 4Ts score consists of the degree and timing of thrombocytopenia, presence of thrombosis, and possibility of other causes of thrombocytopenia [
8]. The gold standard of HIT immunoassays is the serotonin release assay (SRA); however, the SRA is not widely available for use in clinical practice [
9]. As an alternative to the SRA, Kim et al. show that a high OD value (i.e., >0.4) in heparin-PF4 ELISA can be used to diagnose HIT with 93.8% sensitivity and 96.6% specificity [
7].
Several HIT cases are reported to have occurred after patients received vascular catheter heparin flushes. The standard dose of heparin used in flushing intravascular catheters has not been established. Most of those cases involved central vascular catheters with direct heparin flushing; infused quantities were approximately 1,000-2,000 units [
10-
12]. In a prospective argatroban study of 23 patients who developed HIT following exposure to heparin because of vascular catheter flushes, heparin doses were 10-13,000 units. The severity of clinical manifestations in HIT cases has not been correlated with amounts of heparin administered [
13]. In the present case, a total of 7,000 units of heparin mixed into the TPN via peripheral vascular catheters caused HIT. Heparin-supplemented TPN is widely used in clinical practice, because it is proven to reduce the risk of thrombophlebitis as well as maintain vascular catheter patency longer [
14]. Because of the very low dosage of heparin employed in this capacity, recognition of HIT may not be straightforward. In the present case, heparin was not administered as a conventional anticoagulant but instead as a locally acting assisting agent to maintain vascular patency. However, this case highlights an important finding: even a very small amount of heparin mixed into TPN can cause HIT and potentially result in fatal thromboembolism and thrombocytopenia. Early clinical suspicion of HIT and immediate substitution of heparin supplementation with alternative anticoagulation therapy are essential to avoid fatal sequelae in such cases [
6]. It is important to begin the investigation and management of HIT preemptively when a patient with clinical risk factors presents with typical manifestations of HIT, even if the amount of heparin exposure is regarded as negligible.
Go to :


