Abstract
Background
In 2004, the Korean government and blood transfusion community deliberated on the issue of a national blood system reform and agreed to implement a 5-year project (2004-2009) to further improve safety measures. Our study delineates the basis of the current national blood program and analyzes the performance of this 5-year project initiated by the Korean government.
Methods
A performance review of the 5-year project was conducted from May 2009 to February 2010 using various approaches. Numerous data and documentation were collected from the Korean Red Cross and the Korean Centers for Disease Control and Prevention and reviewed by experts. Approximately 20 interviews with representatives of stakeholder groups were conducted to gather information, opinions, and perceptions. We conducted a nationwide field survey on a total of 144 blood donor centers.
Results
Among the 5 major categories of the 5-year project, blood donor recruitment, laboratory testing, and product manufacturing were improved in terms of quality performance. Specifically, government's financial support ensured that the infrastructure of blood donor centers and blood laboratory centers improved. The pivotal role of the government contributed to improvements in the national blood program and enhanced national surveillance for blood safety.
Conclusion
Korea has made a tremendous effort with positive outcomes to provide safety measures for blood products for transfusion in its citizens. In all areas of blood management, from blood donations to transfusions, continuous developments in monitoring safety standards and practices are paramount.
The management and allocation strategies of national blood programs vary according to each country's established ractices [1, 2]. The ability to provide safe and secure blood supply is the cornerstone of transfusion medicine in the health care community. However, a tremendous challenge exists in the field of blood supply as the demands for blood products is rising as a result of increased individual access to health care services and life expectancy. Blood products are not synthetic products but rather a limited resource dependent on donor availability, and have therefore become an expensive resource.
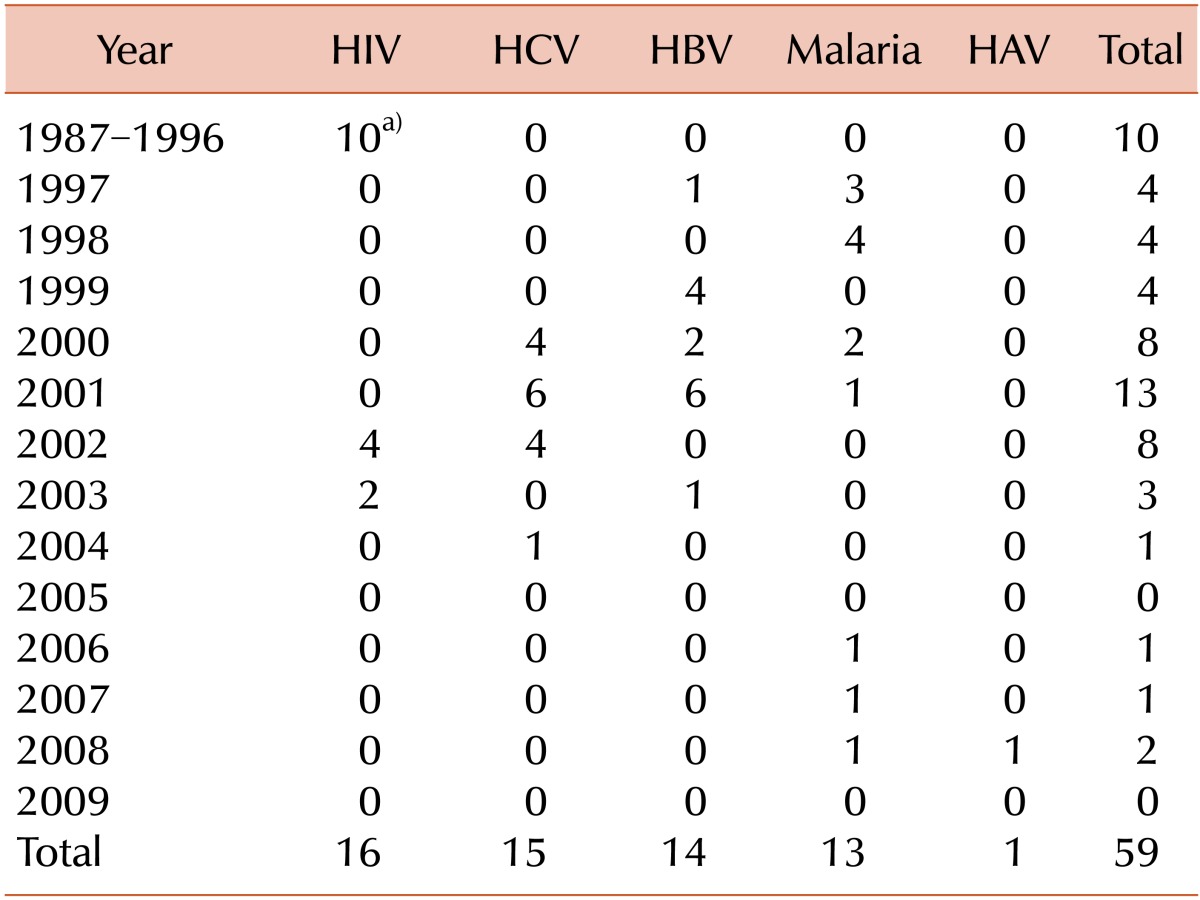
Since 1981, the Korean Red Cross (KRC) has been responsible for establishing quality performance and executing guidelines for the national blood program. However, a 20-year retrospective database study revealed isolated cases of transfusion-transmitted infections (TTI) by human immunodeficiency virus (HIV), hepatitis B virus, and hepatitis C virus (HCV), causing a perception among the public of poor quality blood bank services in Korea. Due to public distrust, an urgent course of action was needed to restore public confidence in the national blood system. A special committee was formed in April 2004 to address the concerns of the citizens and health care community. The 11 committee members consisted of the Deputy Minister for Social Welfare, the chairman of the Korean Society of Blood Transfusion, 5 experts from academic institutions, 2 government officials from the Ministry of Health and Welfare, an official from the Prime Minister's Office, and the director of the KRC Blood Service Headquarters. In 2004, multiple committee hearings, small and large group conferences, and on-site inspections were conducted to gather data on day-to-day operations. Five sectors of concern, 1) blood donor recruitment, 2) blood testing and manufacturing, 3) blood supply and use, 4) blood service expertise enhancement, and 5) government responsibility for the national blood program, were identified as being problematic in the national blood program, and plans to rectify these issues over the following 5 years were announced in September 2004. Following this, the Korean blood transfusion community announced that they would initiate actions to further improve safety measures from 2004.2009.
Our study delineates the basis of the current national blood program and analyzes the data and results of the 5-year project by the Korean government to ensure safe and secure blood supply.
A performance review of the National Blood Safety Improvement Project was conducted from May 2009 to February 2010. It was important that a comprehensive data collection approach, especially from KRC, be established early in the project. After this project was approved by the KRC institutional review board, a total of 66 documents or data were requested and secured. Requested data were classified into 8 categories including improvement of blood donor center facilities, encouragement of registered blood donors, assurance of stable supply of single donor platelets, support of voluntary blood drive, improvement of blood laboratory facilities, development of blood information sharing system, transportation of blood products, and innovation in the organizational structure of the KRC. A total of 20 documents were provided by the division of human blood safety surveillance of the Korea Centers for Disease Control and Prevention (KCDC). Key documents reviewed included national blood collection and utilization surveys, look-back system in transfusion-transmitted infection, inspection results of blood establishments/blood collection centers, educational activities by the government, and research activities of the KCDC.
Interviews were conducted to gather information, opinions, and perceptions. Our team conducted approximately 20 interviews with representatives of the following stakeholder groups: current and past KRC blood services headquarter representatives, government representatives, hospital decision-makers, transfusion specialists, non-profit blood donor organization representatives, and consumer (patient) group representatives. The overall objective of the interviews was to obtain information that would allow an assessment of the performance of the national blood program.
We conducted a field survey on a total of 144 blood donor centers from all over the country between July 2009 and October 2009. The survey was conducted in close collaboration with the KRC and Hanmaum blood center. A 2-person surveyor team visited each blood donor center with the same questionnaires. Major survey items were related to facility (e.g., location, area, parking space, and space composition of each function), management of human resources, equipment, and transportation method of blood products.
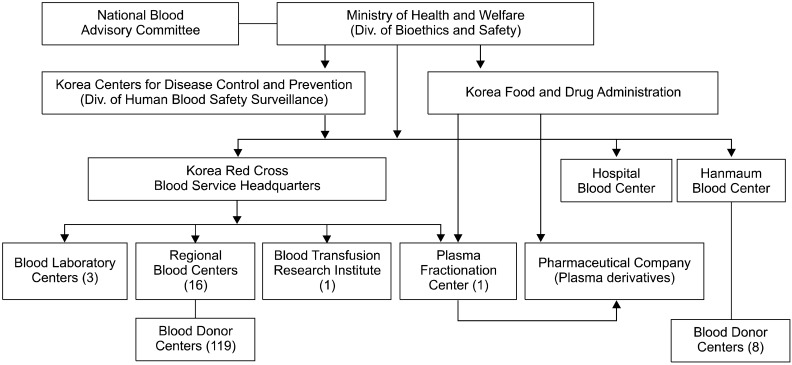
The Ministry of Health and Welfare is responsible for the national blood program in Korea (Fig. 1). With the execution of the 5-year project, 2 departments dedicated to the national blood program were established in the Ministry of Health and Welfare and the KCDC. Specifically, the division of human blood safety surveillance in the KCDC played a key role in the implementation of the 5-year project. The Korea Food and Drug Administration (KFDA) is responsible for regulating the plasma fractionation industry by licensing facilities and products, on-site inspecting licensed plasma fractionators, and issuing and enforcing safety rules. New regulations on organization and operations of the National Blood Advisory Committee have been enacted to strengthen these activities.
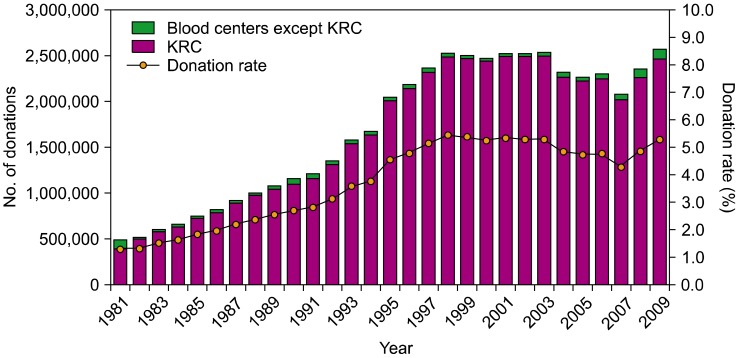
The KRC plays a pivotal role in blood collection in Korea, being involved in tasks such as the manufacturing, testing, and distribution of blood products. They operate through their blood service headquarters, 16 regional blood centers, 3 blood laboratory centers, 1 plasma fractionation center, and 1 blood transfusion research institute. The 16 regional blood centers are located in 8 provinces with 119 blood donor centers. Two alternate blood collection sites in Korea include the hospital blood centers and the Hanmaum blood center, which are private organizations. The total number of donations in 2009 was 2,579,954, of which 2,471,880 (95.8%) were donated through the KRC; blood donation through the Hanmaum blood center and hospital blood centers accounted for only 108,074 donations (4.2%). The proportion of blood units collected at these latter 2 institutions comprised 3.5% of the total blood donated in Korea. The Korean population in 2009 was 48,747,000 people, and 53 of every 1,000 individuals were blood donors (donation rate: 5.3%) (Fig. 2). Because of increasingly stringent donor criteria for the prevention of possible transfusion-transmitted infections, the deferral rate was 19.4% of all donation candidates in 2009 (Fig. 3).
The proposal for the National Blood Safety Improvement Project was divided into 5 main components: blood donor recruitment, laboratory testing, product manufacturing, supply chain, and use of blood products. The major achievements of this commitment are summarized in Table 1. The financial support from the government totaled 101 million US dollars, and the annual blood program operation cost increased to approximately 200 million US dollars over the 5 years.
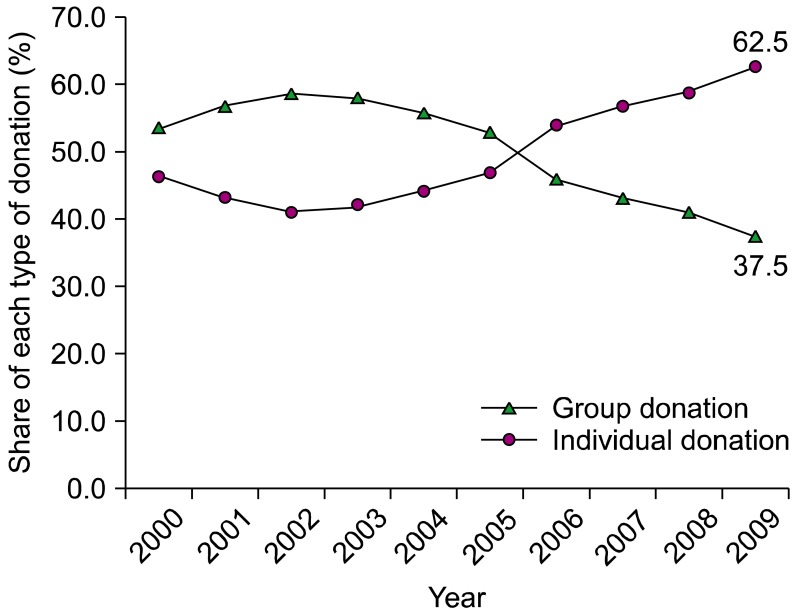
Before the 5-year project, Korea obtained many of its blood products through group donations, usually from students or the military. Many blood donor centers were set up in high-traffic areas to attract more donors, and measures were taken to facilitate the donation process. Among 127 blood donor centers (119 at the KRC and 8 at the Hanmaum blood center), 58 blood donor centers were newly established or remodeled through governmental financial support. Through these efforts, the number of individual donations increased, reaching 62.9% of the total number of donations in 2009 (Fig. 4).
The blood laboratory centers that perform testing for infectious disease markers were consolidated from 7 locations to 3 sites, located in Seoul (capital of Korea), Daejeon (center of the country), and Busan (southernmost large city in the country) (Fig. 5). The following screenings are performed in these laboratories: hepatitis B virus surface antigen (Ag) and anti-human T-lymphotropic virus antibody (Ab) by chemiluminescence immunoassay, anti-human HIV Ab and anti-HCV Ab by enzyme immunoassay, nucleic acid amplification test (NAT) for HIV and HCV, serologic test for syphilis by Treponema pallidum particle agglutination assay, and alanine aminotransferase test by chemistry analyzer. For blood samples collected during the day, testing is performed the same evening in the laboratory.
The Blood Information Sharing System (BISS) network between the KRC and hospital blood centers was completed in 2006 by the KRC. The objective of the system was to provide real-time information on the availability of specific blood products, especially when the hospital seeks a rare blood type. The system allows for immediate exchange of information from any location through mobile devices. Information regarding previous results of infectious disease markers of blood donors who donated at the KRC collection center since 1981 is recorded in the KRC Donor Registry database and available for real-time acquisition by the KRC blood donor centers and hospital blood centers. This process was completed in 2005, and a backup of the system at the national disaster control center was completed in 2009. In addition, the KRC receives donor information from the KCDC database for new enrollment of HIV-, malaria-, Brucella-, and Babesia-infected patients. Furthermore, information has been collected from the Health Insurance Review and Assessment Service (HIRA) and military services databases for data on growth hormone and hepatitis B immunoglobulin medications, as well as medications for baldness, psoriasis, and prostate hypertrophy, among others, for screening after collection.
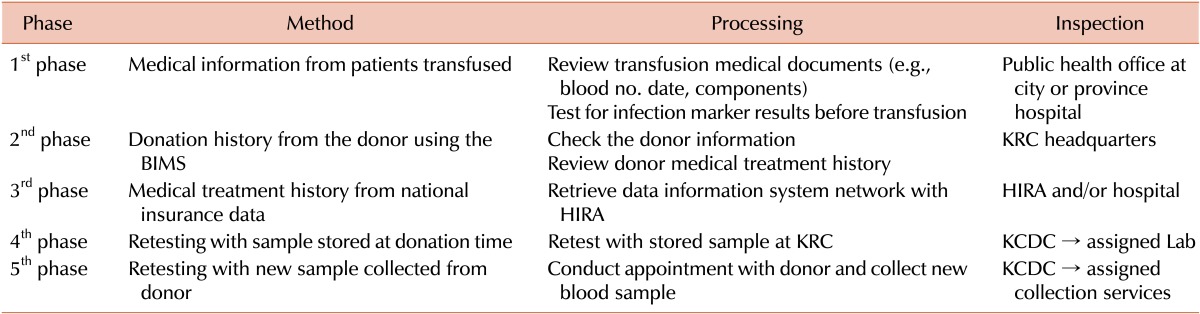
In 2005, the KCDC initiated a retrospective analysis on blood that had been transfused and was under suspicion for causing infections. For suspected cases of TTI, the donor history was carefully inspected. Then, further evaluation assessing whether the donor made multiple donations at the suspected time was conducted. If these evaluations were negative, TTI was ruled out. Since 2005, all tested samples of donated blood have been stored in the KRC storage bank for a period of 10 years. Stored samples undergo repeated testing for infections; if these tests do not yield positive results, TTI is ruled out. If more information is required to confirm TTI, more data regarding the donor are confirmed using medical treatment histories from the health insurance database using the national HIRA network. If TTI still cannot be ruled out, the donor is contacted and a new sample is collected. Table 2 summarizes the review and assessment process for TTI. Because data collection began in 2005, there are some gaps between the transfused and reported dates. As a result of this system, the number of reported cases of infection has decreased each year, and the number of TTIs involving diseases such as hepatitis B, hepatitis C, and HIV have not increased since 2005 (Table 3).
While the government oversees the national blood program in Korea, the specifics of the blood collection, manufacturing of blood products, and testing of blood samples are overseen and executed by the KRC. The national blood program was consigned to the KRC in 1981, with the exception of a few private operations. However, the emergence of 6 cases of transfusion-induced AIDS in 2004 instigated safety concerns from the public [3]. This led to the opening of a private blood establishment, the Hanmaum blood center. Since then, blood products have been collected by 3 main organizations: the KRC, the Hanmaum blood center, and hospital blood centers.
The primary goal of the 5-year project was to restore public trust in blood donation and transfusion. In terms of blood donation, the total number of donations has been restored from 2,087,762 in 2007 to 2,575,954 in 2009. Furthermore, the proportion of individual donations increased from 41.9% in 2003 to 62.5% in 2009. The establishment of new blood donor centers and remodeling of existing blood donor centers through this 5-year project led to many dramatic changes in how blood donation is carried out in Korea. Improved services at blood donor centers including friendly staff and physical infrastructure, easy access and registration, and more comfortable space led to high donor satisfaction. Our field survey revealed higher scores for blood donor centers rebuilt by governmental financial support than for existing blood donor centers.
The second goal of the 5-year project was to ensure the safety of blood products and their transfusion. Numerous actions performed to ensure safety of blood products include consolidation of blood laboratory centers, implementation of laboratory automation systems in blood laboratory centers, implementation of NAT screening for HIV and HCV, implementation of anti-HTLV Ab testing, and establishment of BISS for real-time acquisition of information. As a result of this project, there have been no newly documented cases of transfusion-transmitted HIV, HCV, or HBV infection.
In conclusion, Korea has made a tremendous effort with positive results to offer safety measures for blood products for transfusions in its citizens. In all aspects, from blood donations to transfusions, continued developments in monitoring safety standards and practices are paramount in each of the existing settings: the KRC and private blood establishments (including hospital blood centers and the Hanmaum blood center). We hope that the government will continue their initiative to ensure safety in national blood supply and services.
Notes
References
1. World Health Organization. Global blood safety and availability facts and figures from the 2007 blood safety, 2009. Geneva, Switzerland: World Health Organization;2009. Accessed October 7, 2010. at http://www.who.int/bloodsafety/global_database/en/index.html.
2. Farrugia A, Penrod J, Bult JM. Payment, compensation and replacement-the ethics and motivation of blood and plasma donation. Vox Sang. 2010; 99:202–211. PMID: 20576023.
3. Cho JE. National blood management system and the direction of government policy in Korea. Korean J Hematol. 2010; 45:81–83. PMID: 21120182.

Fig. 3
The changes in the number of presenting donors and deferred donors and the donor deferral rate in the past 27 years.
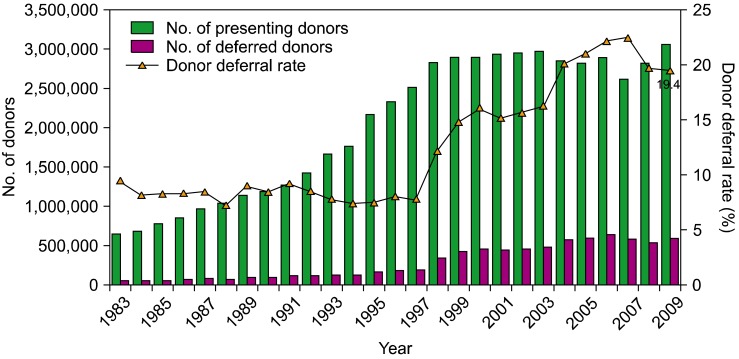
Table 1
Achievements of the national blood safety improvement project.
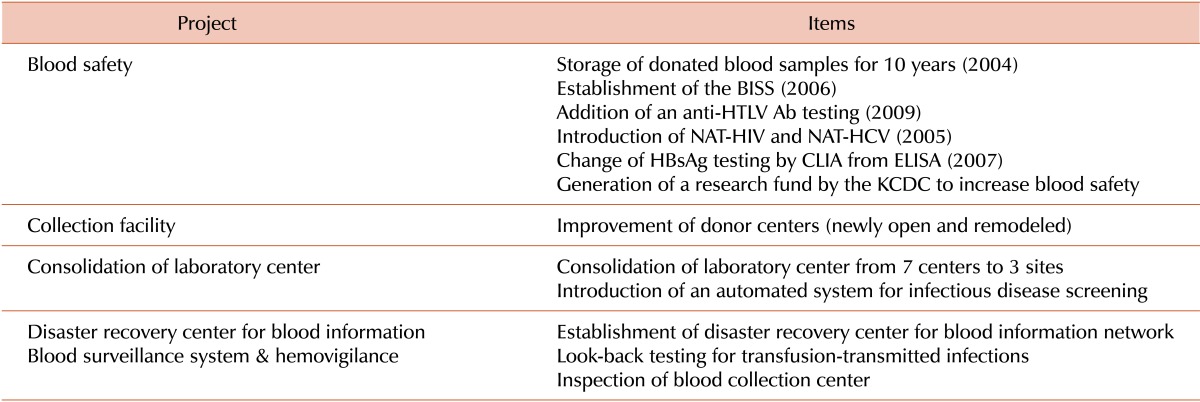
Abbreviations: BISS, blood information sharing system; Anti-HTLV Ab, anti-human T-lymphotropic virus antibody; NAT-HIV, nucleic acid amplification test for human immunodeficiency virus; NAT-HCV, nucleic acid amplification test for hepatitis C virus; HBsAg, hepatitis B surface antigen; CLIA, chemiluminescence immunoassay; ELISA, enzyme-linked immunosorbent assay; KCDC, Korea Centers for Disease Control and Prevention.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download