Abstract
Background
Most children with acute lymphoblastic leukemia (ALL) receive blood transfusions. Transfusions may affect ALL outcomes through transfusion-related immunomodulation (TRIM).
Methods
We analyzed overall survival (OS) and event-free survival (EFS) in relation to leukocyte reduced and irradiated (LR/IRR) blood products transfused during the induction phase in 136 children with ALL. Hazard ratios (HRs) for death and relapse were estimated through Cox regression analysis.
Results
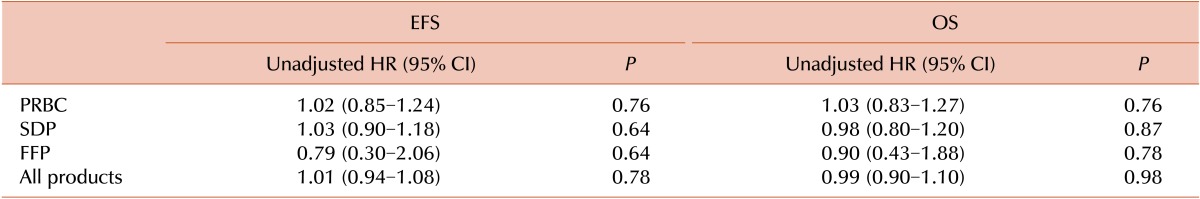
One hundred and twenty patients (89%) were transfused with packed red blood cells (PRBCs) and 79 (58%) with single donor platelets (SDPs). The median number of transfusions was 2 (interquartile range [IQR]=1-3 events) and 1 (IQR=0-3 events) for PRBCs and SDPs, respectively. Patients who had white blood cell (WBC) count >50,000×109/L, were classified as high risk according to the high National Cancer Institute criteria, displayed a T cell phenotype, or were minimal residual disease-positive at end of induction were more likely to receive >3 transfusions during induction (P=0.001, 0.002, 0.03, and 0.01, respectively). In univariate analysis, PRBC, SDP, and fresh frozen plasma transfusions did not have any significant association with relapse or death. For PRBC transfusions, the HRs for EFS and OS were 1.02 (95% CI, 0.85-1.24; P=0. 76) and 1.03 (95% CI, 0.83-1.27; P=0.76), respectively. For SDP transfusions, HRs were 1.03 (95% CI, 0.90-1.18; P=0.64) and 0.98 (95% CI, 0.80-1.20; P=0.87) for EFS and OS, respectively.
Allogeneic blood transfusion exposes the recipient to a wide variety of soluble and cell-mediated alloantigens, cytokines, and other cellular components; therefore, it can be considered as a form of allogeneic transplantation. Allogeneic blood transfusions are associated with immune-related effects in the form of allo-immunization or immune suppression [1]. This complex interaction between the transfused component and the host immune system is known as the transfusion-related immunomodulation (TRIM). TRIM was originally identified to produce a beneficial effect in patients by increasing renal allograft survival [2]. However, the TRIM effect was later associated with tumor growth, bacterial infection, postoperative mortality, and organ dysfunction [3, 4].
The exact mechanism of TRIM is poorly identified in the literature, but its immunomodulation could be a result of inhibition of immunologic effector cells or stimulation of suppressor cells [1]. Some of the possible effects include downregulation of the immune function by decreasing cytokine production, mitogen response, T helper cells, natural killer cells, lymphocyte numbers, cell-mediated cytotoxicity, and an increase in T suppressor cell number and function [5].
Cancer continues to be one of the major indications for blood transfusion [6], and children with acute lymphoblastic leukemia (ALL) are no exception. Previous studies have demonstrated that most children with ALL require blood transfusions during therapy, mainly during the induction period [7, 8]. It has been proposed that TRIM effects during this critical induction phase might adversely contribute to ALL patient outcomes through immune suppression. Two previous pediatric studies reporting the TRIM effect in ALL patients produced conflicting results [7, 8]. Early work by Freiberg et al. [7] suggested that TRIM is unlikely in childhood ALL and that the poor outcome associated with transfusions is likely a secondary affect due to disease severity and reduction in chemotherapy doses. In contrast, Jaime-Pérez et al. [8] suggested that TRIM represents an independent adverse prognostic factor in childhood ALL. The blood products used by Jaime-Pérez et al. [8] were leukocyte reduced only, but were both leukocyte reduced and irradiated in the study by Freiberg et al. [7].
Children with ALL are severely immunosuppressed during the intensive induction phase of therapy, during which they receive the majority of blood product transfusions. Additional immune suppression during this critical period might alter their clinical outcome by decreasing the ability of the patient's immune system to eradicate residual leukemic clones. The aim of our study was to determine associations between irradiated and leukocyte-reduced blood products administered during the intensive induction phase of therapy and clinical outcome in children with ALL.
A retrospective study was conducted on children (age, <19 years) diagnosed with ALL at King Hussein Cancer Center in Jordan from 2007 to 2009. The clinical and laboratory features of 136 children were analyzed. Data were collected through medical chart reviews and the Pediatric Oncology Networked Database (POND). POND is a web-based data collection tool developed by St. Jude Children's Research Hospital in 2004, where data on diseases are entered prospectively and updated on a monthly basis.
The treatment protocol and response assessment method have been previously published [9]. The treatment protocol consisted of a combination of the St. Jude Children's Research Hospital Total XIII and Total XV ALL protocols. The 6-week induction phase was similar for all patients and was composed of 7 drugs (prednisolone, vincristine, daunorubicin, 6-mercaptopurine, cyclophosphamide, cytarabine, and L-asparaginase). Patients subsequently undertook an 8-week consolidation phase followed by continuation therapy on 3 separate regimens. The total duration of treatment for all risk groups was 2.5 years for females and 3 years for males. Treatment response was assessed by minimal residual disease (MRD) and morphology on day 15 and at the end of the induction period. A MRD panel was selected according to the patient's previously determined immunophenotype. The following monoclonal antibody combinations, CD10/CD19/CD45/CD34 and CD20/CD45/CD19, were used to immunophenotype each patient's marrow sample and the combination of CD33/CD13/CD45/CD19 was utilized, if the patient's leukemic cells previously expressed a myeloid marker. CD45 and side-scatter were used for gating strategy. The result was reported negative, if <0.01% of cells stained positive for the markers, and positive results were reported as a percentage of the total population of cells collected. MRD evaluation was performed on day 15 of induction and was repeated on completion of the induction period.
The number, frequency, time, and type of any blood product transfusion and its preparation method were captured for all patients through electronic laboratory database. The transfusion of packed red blood cells (PRBCs), single donor platelets collected by apheresis (SDPs), or fresh frozen plasma (FFP) of 10-15 mL/kg was considered as a transfusion event. Other transfusion events such as cryoprecipitate or blood-derived platelet concentrate were negligible and not considered for analysis. The transfusion decisions were based on the treating physicians' clinical judgment and guided by universal transfusion guidelines. All blood products were leukocyte reduced (achieving leukocyte numbers well below 5×105) and irradiated with 2,500 cGy for 3-5 minutes.
Demographic and clinical characteristics were presented as means, medians, and ranges for continuous variables, or frequencies and percentages for categorical variables. The relationships between known adverse prognostic features and the transfusion requirements during induction were determined by using logistic regression analysis. Events considered for survival analysis were relapse, death from any cause, or secondary cancer. Hazard ratios (HRs) for death and events were estimated through uni- and multivariate Cox regression analysis. Overall survival (OS) and event-free-survival (EFS) curves were presented using the Kaplan-Meier method. Comparisons between OS and EFS curves were performed using the Log rank test. A two-sided P value of ≤0.05 was considered statistically significant. All analyses were carried out using SPSS for Windows v.16.0 (SPSS Inc., Chicago, IL, USA).
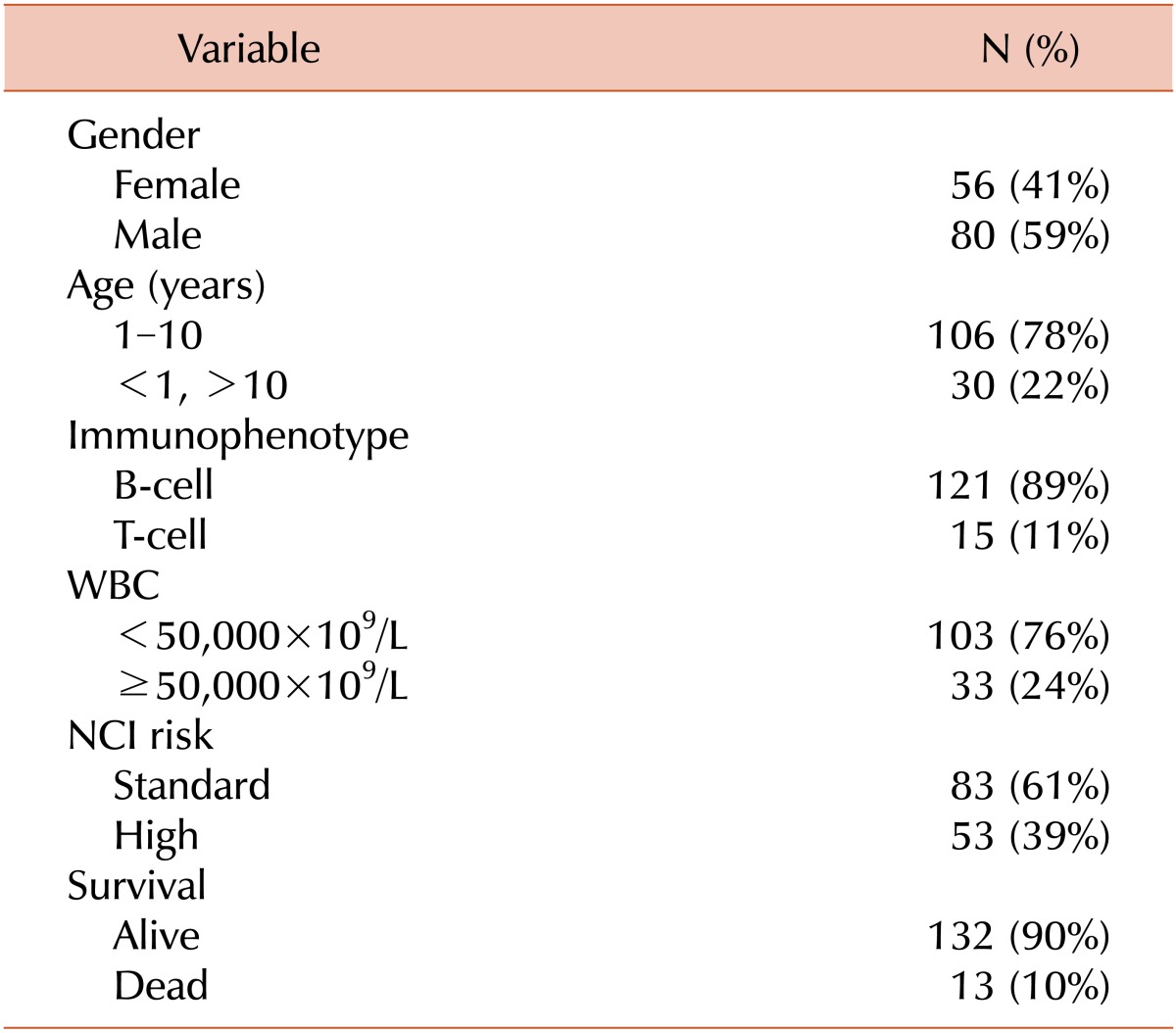
During the study period, 136 patients were analyzed with a median age of 5 years (range, 0-18 years). There were 80 (59%) males and 56 (41%) females. One hundred and twenty one (89%) had pre-B ALL and 15 (11%) had T cell ALL. The major clinical characteristics of this cohort are summarized in Table 1. After a median follow-up of 35.5 months (range, 2-54), the estimated 4-year EFS and OS were 67% and 87%, respectively.
During the critical induction period of chemotherapy 121 (89%) patients were transfused with PRBCs, 79 (58%) with SDPs, and 15 (11%) with FFP. The median number of PRBC and SDP transfusions for each was 2 (mean, 2; range, 0-21) and 1 (mean, 2; range, 0-25), respectively.
Univariate regression analysis showed that patients who had a WBC >50,000×109/L, were classified as a high-risk group based on NCI criteria (age, <1 year or >9 year, and WBC >50,000×109/L), displayed T cell phenotype, or were MRD positive at the end of induction were more likely to receive >3 transfusions (the median of combined transfusions) during the induction phase (P=0.001, 0.002, 0.03, and 0.01, respectively). In a multivariate regression analysis model (including WBC count, immunophenotype, and MRD status), only WBC >50,000×109/L independently predicted a need for >3 transfusions during induction period (P=0.01).
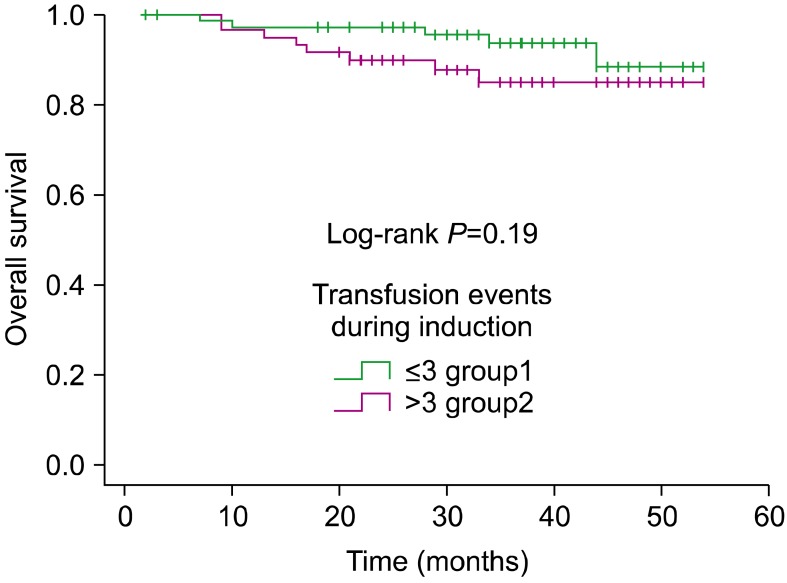
In univariate analysis, PRBC, SDP, and FFP transfusions did not have any significant association with adverse outcome, as summarized in Table 2. For PRBC, the HRs for EFS and OS were 1.02 (95% CI, 0.85-1.24, P=0. 76) and 1.03 (95% CI, 0.83-1.27, P=0.76), respectively. For SDP, the HR was 1.03 (95% CI, 0.90-1.18, P=0.64) and 0.98 (95% CI: 0.80-1.20, P=0.87) for EFS and OS, respectively. When analyzing the influence of the absolute number of transfused blood products on survival, patients who received <3 units (less than the median) had a 4-year EFS rate of 71% (SE=0.12), whereas those who received >3 units had a 4-year EFS rate of 50% (SE=0.16) (P=0.12) (Fig. 1). When considering OS, patients who received <3 units had a 4-year OS rate of 88% (SE=0.05), whereas those who received >3 units had a 4-year OS of 85% (SE=0.05) (P=0.19) (Fig. 2).
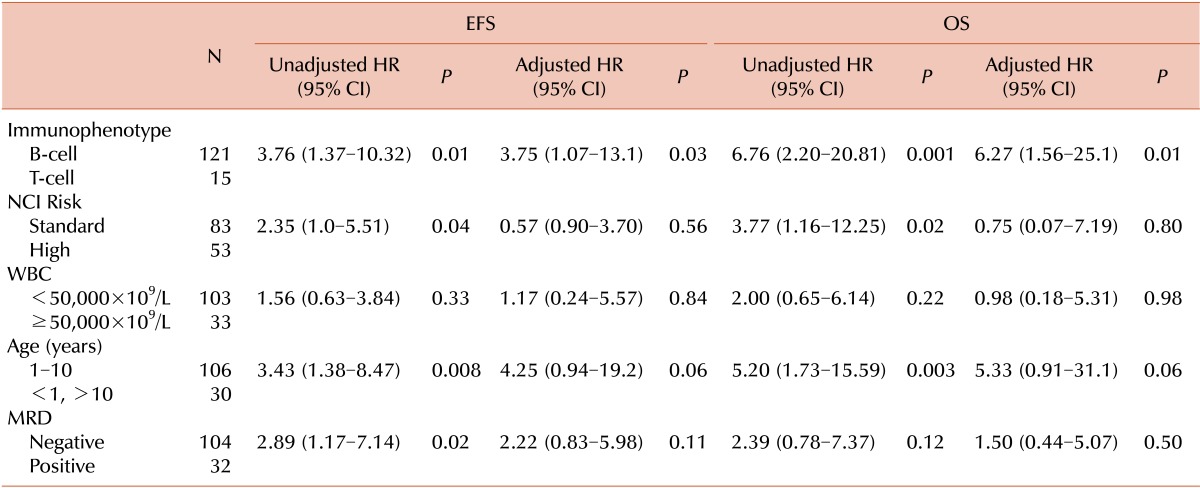
Other known prognostic factors in childhood ALL were examined in univariate and multivariate analyses (Table 3). T cell phenotype, high NCI risk group, age >10 years, and positive MRD status at completion of induction were significantly predictive of poor EFS. T cell phenotype, high NCI risk group, and age >10 years were significantly predictive of poor OS. The number of transfusion events itself during induction phase did not predict MRD status at the end of induction (P=0.41).
When we analyzed the number of transfusions from diagnosis till 1 month before the adverse event, transfusions also failed to show any prognostic effect on clinical outcome of childhood ALL. For example, for the combined transfusions, the HR for EFS and OS were 0.99 (95% CI, 0.95-1.03; P=0.77) and 0.97 (95% CI, 0.92-1.03; P=0.39), respectively. After analyzing events of transfusions till just before relapse or death, we found that transfusion events were predictive of EFS and OS (HR, 1.04; 95% CI, 1.01-1.07; P=0.003) and (HR, 1.04; 95% CI, 1.01-1.07, P=0.004), respectively.
The possible beneficial effect of TRIM was initially reported in renal transplantation studies, where it was suggested to improve renal graft survival [2]. Later, the adverse effects of TRIM were studied and shown to contribute to cancer growth, infections, and postoperative mortality [3, 4]. The association between TRIM and cancer progression was first reported in colon cancer by Lewis Burrows and Paul Tartter in 1982 [10], and subsequently in other cancer types, including breast cancer [11], head and neck cancer [12], lung cancer [13], soft tissue cancer [14], stomach cancer [15], and adult leukemia [16]. Although the effect of TRIM has been reproduced in multiple retrospective studies, a few randomized controlled trials have failed to establish the same [17-19]. It is not ethical to perform randomized trials where patients are randomly allocated to either never or always receive blood transfusions. However, it is possible to prospectively randomize patients who have received transfusions consisting of different blood products (i.e. leukoreduced vs. non-leukoreduced). The 3 previous randomized trials included patients undergoing colorectal cancer resection, and the findings were combined into 2 meta-analyses [20, 21]. In the first meta-analysis, the summary odds ratio (OR), averaged across the 3 studies, of cancer recurrence in the allogeneic transfusion group compared to the control group, was 1.04 (95% CI, 0.81-1.35; P>0.05) [20]. In the second meta-analysis, the summary OR of death due to cancer recurrence was 0.98 (95% CI, 0.76-1.26; P>0.05.) [21]. In contrast, experimental animal models strongly suggest a tumor-promoting effect and showed that such an effect can be reduced by depletion of leukocytes from the blood transfusion product [22]. Cancer patients, including children with ALL, are major consumers of different blood products during their treatment, particularly in the induction phase [7, 8]. The induction period is critical in ALL therapy, and any additional immune suppression could adversely affect patient outcome.
The early work on TRIM in childhood ALL was performed by Freiberg et al. [7]. They retrospectively analyzed 358 children with ALL from 1984 to 1988. Almost all children required blood transfusions, mainly during induction. Since all patients in the study who received >50 units had poor outcome, the number of units was predictive of poor outcome in multivariate analysis. When those patients were excluded from the analysis, the number of units was only predictive in univariate analysis. The authors concluded that the high number of transfusions was an epiphenomenon that reflected other factors (like disease severity and chemotherapy reduction), and that a TRIM effect in these patients was unlikely.
Recently, Jaime-Pérez et al. [8] published their results regarding TRIM effects in childhood ALL. They retrospectively studied 108 children with ALL diagnosed between 2000 and 2009 for possible TRIM. Around 90% of patients were transfused, predominantly during the induction phase of therapy. In multivariate analysis, transfusion of >5 units of PRBCs was predictive of death and relapse (P=0.003 and 0.01, respectively). For whole blood-derived platelets concentrate (PC), maximum effect on death was seen with >30 transfused units (P=0.001). Even after the exclusion of outliers, data still supported a TRIM effect. The authors concluded that TRIM effect may be an independent prognostic factor in children with ALL.
One of the explanations of the discrepancy between the previous 2 studies might be the preparation method. The blood products in the study by Freiberg et al. [7] were leukocyte reduced and irradiated, whereas only leukocyte-reduced products were used in the study by Jaime-Pérez et al [8]. Irradiation might prevent mononuclear cell proliferation, which is necessary for transfusion-associated microchimerism (TA-MC). TA-MC occurs in severely injured patients. Such patients develop massive inflammatory response leading to immune suppression, and transfused blood products with replication-competent leukocytes can lead to TA-MC [11]. During the intensive induction phase of therapy for children with ALL, a similar effect may happen with blood transfusion. Jaime-Pérez et al. [8] suggested that a TA-MC phenomenon in combination with TRIM might explain the reduced survival of ALL patients receiving blood transfusions; however, this suggestion lacks strong evidence and remains as a hypothesis. The other possible explanation for the discrepancy in these 2 studies might be the study design itself, as the significance of the results can vary dramatically depending on the inclusion period of the study. Analysis of all events of transfusions till just before relapse or death showed that PRBC events were predictive of EFS and OS in univariate and multivariate analysis. This is easy to explain, since the majority of patients with undiagnosed relapse present with marrow suppression, and most require transfusions as a result of relapse rather than being a cause of it.
In our study, we failed to show any independent effect of blood transfusions during the induction period on the clinical outcome of childhood ALL. The most critical period of immune suppression that could theoretically affect outcome is during the intensive induction phase. The detailed analysis of different types of transfusion events during the induction phase failed to show any independent adverse effect on outcomes. It is important to note that the role of irradiation in eliminating such TRIM effects, as proposed by Jaime-Pérez et al. [8], could not be assessed in our cohort since irradiation is universally performed for all cellular blood products at our institution.
Our study had 4 major limitations. First, because of the retrospective design, a cause and effect relationship could not be proven. Second, medical charts have limited information that prevents assessment of additional important confounders, such as undocumented delay, reduction in chemotherapy, or patient compliance. Third, the transfusion decision was not standardized but depended on the medical judgment of the treating physician; this may represent a possible source of bias. Fourth, our data analysis did not consider the age of blood products transfused; this factor has been suggested to have a tumor-promoting effect [23].
In summary, our study examined the possibility of TRIM effects in childhood ALL. Our data showed that most children are exposed to blood products during the induction phase of their treatment, but without any adverse effect on clinical outcome (induction failure, relapse, or death). Patients with high-risk characteristics were more likely to receive transfusion, which could be due to disease severity and therapy intensity. The impact of special treatment of the blood products, such as leukocyte reduction and irradiation, on patient outcome could not be assessed in this cohort since these preparations were standardized for all transfusions at our center. Given the limitations of our current retrospective study, this lack of TRIM effect in children with ALL should be further validated in a prospective manner.
References
1. Blumberg N, Triulzi DJ, Heal JM. Transfusion-induced immunomodulation and its clinical consequences. Transfus Med Rev. 1990; 4(4 Suppl 1):24–35. PMID: 2134638.

2. Opelz G. Current relevance of the transfusion effect in renal transplantation. Transplant Proc. 1985; 17:1015–1021.
3. Landers DF, Hill GE, Wong KC, Fox IJ. Blood transfusion-induced immunomodulation. Anesth Analg. 1996; 82:187–204. PMID: 8712400.

4. Blajchman MA. Transfusion immunomodulation or TRIM: what does it mean clinically? Hematology. 2005; 10(Suppl 1):208–214. PMID: 16188675.

5. Blumberg N, Heal JM. Transfusion and recipient immune function. Arch Pathol Lab Med. 1989; 113:246–253. PMID: 2645852.
6. Friedman BA, Burns TL, Schork MA. A study of blood utilization by diagnosis, month of transfusion, and geographic region of the United States. Transfusion. 1979; 19:511–525. PMID: 92077.

7. Freiberg AS, Hancock ML, Kunkel KD, Rivera GK, Crist WM. Transfusions and risk of failure in childhood acute lymphoblastic leukemia. Leukemia. 1994; 8:1220–1223. PMID: 8035615.
8. Jaime-Pérez JC, Colunga-Pedraza PR, Gómez-Almaguer D. Is the number of blood products transfused associated with lower survival in children with acute lymphoblastic leukemia? Pediatr Blood Cancer. 2011; 57:217–223. PMID: 21671359.

9. Halalsheh H, Abuirmeileh N, Rihani R, Bazzeh F, Zaru L, Madanat F. Outcome of childhood acute lymphoblastic leukemia in Jordan. Pediatr Blood Cancer. 2011; 57:385–391. PMID: 21360658.

10. Burrows L, Tartter P. Effect of blood transfusions on colonic malignancy recurrent rate. Lancet. 1982; 2:662. PMID: 6125797.
11. Hoe NY, Herman KJ, Hermann RE, Medendorp SV. Perioperative blood transfusion and survival of breast cancer patients after modified radical mastectomy. Cleve Clin J Med. 1991; 58:515–519. PMID: 1752033.

12. Böck M, Grevers G, Koblitz M, Heim MU, Mempel W. Influence of blood transfusion on recurrence, survival and postoperative infections of laryngeal cancer. Acta Otolaryngol. 1990; 110:155–160. PMID: 2386031.
13. Little AG, Wu HS, Ferguson MK, et al. Perioperative blood transfusion adversely affects prognosis of patients with stage I non-small-cell lung cancer. Am J Surg. 1990; 160:630–632. PMID: 2174651.

14. Rosenberg SA, Seipp CA, White DE, Wesley R. Perioperative blood transfusions are associated with increased rates of recurrence and decreased survival in patients with high-grade soft-tissue sarcomas of the extremities. J Clin Oncol. 1985; 3:698–709. PMID: 3998786.

15. Kampschöer GH, Maruyama K, Sasako M, Kinoshita T, van de Velde CJ. The effects of blood transfusion on the prognosis of patients with gastric cancer. World J Surg. 1989; 13:637–643. PMID: 2683404.

16. Blumberg N, Heal JM, Liesveld JL, Phillips GL, Rowe JM. Platelet transfusion and survival in adults with acute leukemia. Leukemia. 2008; 22:631–635. PMID: 17805333.

17. Heiss MM, Mempel W, Delanoff C, et al. Blood transfusion-modulated tumor recurrence: first results of a randomized study of autologous versus allogeneic blood transfusion in colorectal cancer surgery. J Clin Oncol. 1994; 12:1859–1867. PMID: 8083709.

18. Busch OR, Hop WC, Hoynck van, Marquet RL, Jeekel J. Blood transfusions and prognosis in colorectal cancer. N Engl J Med. 1993; 328:1372–1376. PMID: 8292113.

19. Houbiers JG, Brand A, van de Watering LM, et al. Randomised controlled trial comparing transfusion of leucocyte-depleted or buffy-coat-depleted blood in surgery for colorectal cancer. Lancet. 1994; 344:573–578. PMID: 7914960.

20. Vamvakas EC. Transfusion-associated cancer recurrence and postoperative infection: meta-analysis of randomized, controlled clinical trials. Transfusion. 1996; 36:175–186. PMID: 8614970.

21. McAlister FA, Clark HD, Wells PS, Laupacis A. Perioperative allogeneic blood transfusion does not cause adverse sequelae in patients with cancer: a meta-analysis of unconfounded studies. Br J Surg. 1998; 85:171–178. PMID: 9501809.

22. Bordin JO, Bardossy L, Blajchman MA. Growth enhancement of established tumors by allogeneic blood transfusion in experimental animals and its amelioration by leukodepletion: the importance of the timing of the leukodepletion. Blood. 1994; 84:344–348. PMID: 8018929.

23. Atzil S, Arad M, Glasner A, et al. Blood transfusion promotes cancer progression: a critical role for aged erythrocytes. Anesthesiology. 2008; 109:989–997. PMID: 19034095.

Fig. 1
Kaplan-Meier analysis of event-free survival according to the number of transfusion events during induction.
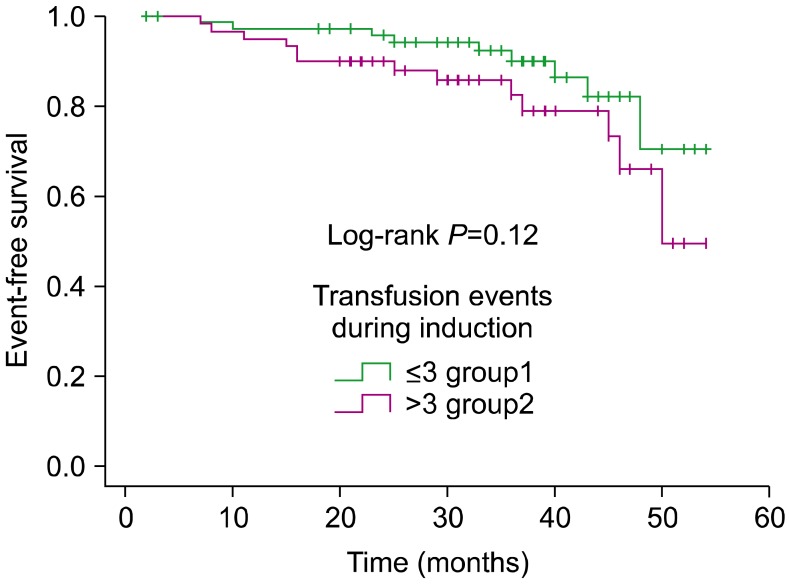
Fig. 2
Kaplan-Meier analysis of overall survival according to the number of transfusion events during induction.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download