INTRODUCTION
Polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (PMF) are subtypes of Philadelphia chromosome-negative myeloproliferative neoplasm (Ph
-MPN) [
1]. The Janus kinase 2 (
JAK2) V617F mutation is the most common molecular abnormality in Ph
-MPN and is found in more than 97% of patients with PV and approximately 50% of patients with either ET or PMF [
1-
4].
According to the revised World Health Organization (WHO) classification of Ph
-MPN, the 3 different disease subtypes show considerable overlap in terms of their clinical presentation, disease course, and associated bone marrow histopathology. Bone marrow histopathology is recommended for discriminating among the subtypes of Ph
-MPN [
5]. Although the
JAK2 V617F mutation is recommended as an essential clonal marker for the diagnosis of Ph
-MPN, its presence alone cannot discriminate among the 3 subtypes of Ph
-MPN because all 3 share this point mutation. Moreover, it is not yet known how a single mutation can result in 3 different disease subtypes that differ in their clinical presentation and prognosis. The prognosis is the best for ET and the worst for PMF.
Recently, real-time quantitative PCR (RQ-PCR) methods that determine the allele burden of
JAK2 V617F have been developed to monitor the disease course in Ph
-MPN patients who carry the
JAK2 V617F mutation. Several studies, focusing on whether differences in the allele burden of
JAK2 V617F might explain the different disease phenotypes associated with subtypes of Ph
-MPN, have shown a higher
JAK2 V617F allele burden in PV than in ET [
6-
8]. A recent study showed that a
JAK2 V617F allele burden >50% favors a diagnosis of prefibrotic PMF rather than ET [
9]. However, the potential of the
JAK2 V617F allele burden to discriminate the subtypes of Ph
-MPN has never been systematically evaluated.
In this study, we performed RQ-PCR to detect the allele burden of JAK2 V617F and evaluated the feasibility of using this as a criterion for discriminating among the subtypes of Ph-MPN.
MATERIALS AND METHODS
Sample collection
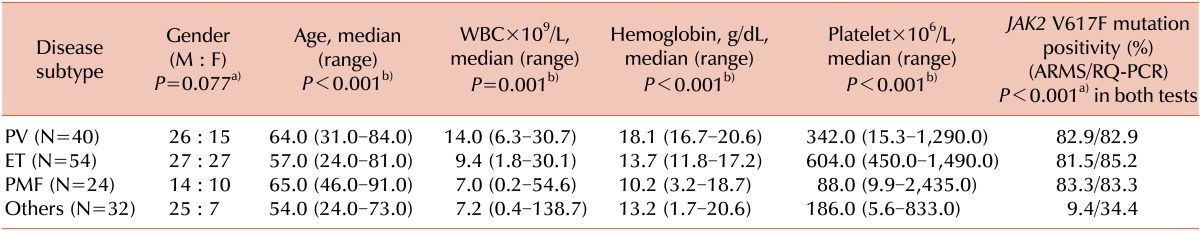
We enrolled 151 patients diagnosed with Ph-MPN at the Asan Medical Center between January 2008 and July 2011. We obtained 70 peripheral blood (PB) and 81 bone marrow (BM) aspiration samples from these patients at initial diagnosis. The diagnosis of Ph-MPN was based on BM histopathology performed by an expert in this area. The numbers of patients diagnosed with PV, ET, PMF, and other subtypes of Ph-MPN were 41, 54, 24, and 32, respectively. Patients diagnosed as secondary Ph-MPN, such as post-ET or PV-PMF, were excluded from the study population to minimize the effect of a primary disorder on the secondary disorder.
Analysis of the JAK2 V617F mutation by allele-specific PCR
Mutation analysis of JAK2 V617F was initially performed using allele-specific PCR based on Amplification Refractory Mutation System (ARMS) technology. Genomic DNA was extracted from each PB and BM aspiration sample at diagnosis, using a QIAamp DNA Mini Kit (QIAGEN Inc., Valencia, CA, USA). After adding 3 µL of primer mixture to 20 µL of PCR master mixture, we added 2 µL of sample DNA (10-20 ng/µL) to bring the total volume to 25 µL. The wild-type allele was amplified using a forward primer with the sequence 5'-TCC TCA GAA CGT TGA TGG CAG-3' and a reverse primer with the sequence 5'-ATT GCT TTC CTT TTT CAC AAG AT-3'. The mutant allele was amplified using a forward primer with the sequence 5'-GCA TTT GGT TTT AAA TTA TGG AGT ATA TG-3' and a reverse primer with the sequence 5'-GTT TTA CTT ACT CTC GTC TCC ACA AAA-3'. The PCR conditions used were as follows: heating at 94℃ for 15 min (initial denaturation), then 34 cycles of 94℃ for 30 s (denaturation), 58℃ for 45 s (annealing), and 72℃ for 45 s (extension), followed by a final extension at 72℃ for 4 min. The amplified products were electrophoresed on agarose gels and visualized using ethidium bromide staining. The wild-type phenotype was scored by observation of a single 229-bp fragment only, homozygous mutations were scored by observation of a single 279-bp fragment only, and heterozygosity was identified by the presence of both a 229-bp fragment and a 279-bp fragment, irrespective of band signal strength.
Quantification of the allele burden of the JAK2 V617F mutation
For quantitative analysis of the allele burden of the JAK2 V617F mutation, we performed RQ-PCR using JAK2 MutaQuant™ (Ipsogen Inc., New Haven, CT). Five microliters of genomic DNA was added to 20 µL of the RQ-PCR premix solution (V617F or wild type) in each well. The V617F RQ-PCR premix solution included 12.5 µL of TaqMan Universal PCR master mix, 1 µL of IPSOGEN PPM-VF primers and probe mix, and 6.5 µL of nuclease-free water. Wild-type RQ-PCR premix solution included 12.5 µL of TaqMan Universal PCR master mix, 1 µL of IPSOGEN PPM-wild-type primers and probe mix, and 6.5 µL of nuclease-free water. Twenty-five microliters of solution was used per well for the RQ-PCR reaction program. The RQ-PCR conditions used were as follows: 50℃ for 2 min, heating at 95℃ for 10 min, and 50 cycles of 95℃ for 15 s and 63℃ for 90 s. The RQ-PCR was performed using a LightCycler 480 instrument (Roche Diagnostics, Mannheim, Germany). Standard curves for both V617F and wild type were constructed using either a V617F or a wild-type plasmid of known value, provided by the manufacturer. The equation was calculated for each curve, and these equations were used to calculate the copy number of V617F and wild-type alleles in unknown samples. The allele burden of JAK2 V617F is expressed as the percentage of V617F copies compared with the sum of V617F and wild-type copies. We determined the level of concordance between the results of the RQ-PCR and ARMS assays and also compared the allele burden of JAK2 V617F among to the subtypes of Ph-MPN.
Statistical analysis
The Kruskal-Wallis test was performed to evaluate whether the allele burden of JAK2 V617F mutation differed significantly among the 3 different disease categories. It was also used for the comparison of demographic and laboratory test results among to the disease subtypes. The Mann-Whitney U test was performed to compare the allele burden of JAK2 V617F mutation among subgroups of Ph-MPN. The Chi-squared test was used for the comparison of gender and JAK2 V617F mutation positivity among disease subtypes. Cohen's kappa coefficient was calculated to determine the statistical measure of agreement between the 2 test results. For analysis of the correlation between laboratory results and allele burden of the JAK2 V617F mutation, Pearson correlation analysis was performed, and correlation coefficients (γ) were obtained. For all analyses, the tests were 2-tailed, and P values≤0.05 were considered statistically significant. All calculations were performed using SPSS 13.0.1 for Windows (SPSS Inc, Chicago, IL).
DISCUSSION
For the diagnosis of Ph
-MPN, the revised WHO classification emphasizes both BM histopathologic findings and presence of
JAK2 mutations. Although each subtype of Ph
-MPN has distinct histopathologic features, discriminating among PV, ET, and PMF based exclusively on histopathologic BM findings is sometimes difficult. The primary challenges relate to obtaining sufficient volumes of specimens (owing to "dry tap" in cases with severe myelofibrosis) and overlap between the histopathologic features of each subtype of Ph
-MPN as the disease progresses. Differentiating among specific subtypes of Ph
-MPN can also be challenging. For instance, it can be difficult to differentiate between the prefibrotic phase with associated thrombocytosis in PMF and ET. Differentiating between these 2 entities is clinically important because prefibrotic PMF is much more likely to progress to myelofibrosis than ET [
5,
10]. Therefore, more objective and comprehensive criteria are needed to discriminate among the various causes of Ph
-MPN.
Until recently, most of the studies that focused on the role of
JAK2 V617F mutation in MPN emphasized the diagnostic importance of the mutation in MPN. Few studies have addressed the phenotypic characteristics associated with the
JAK2 V617F mutant allele burden. The availability of methods involving RQ-PCR to analyze the allele burden of
JAK2 V617F has opened the way for evaluating the clinical relevance of the allele burden of the
JAK2 V617F mutation as a criterion useful for discriminating among the subtypes of Ph
-MPN. Estimating the allele burden of the
JAK2 V617F mutation also enables precise interpretation of its impact on the clinical phenotype [
11]. Few studies have comprehensively evaluated the
JAK2 V617F allele burden as a criterion to discriminate among the 3 disease subtypes of Ph
-MPN. Instead, all these studies have compared the
JAK2 V617F allele burden between PV and ET only [
6-
8].
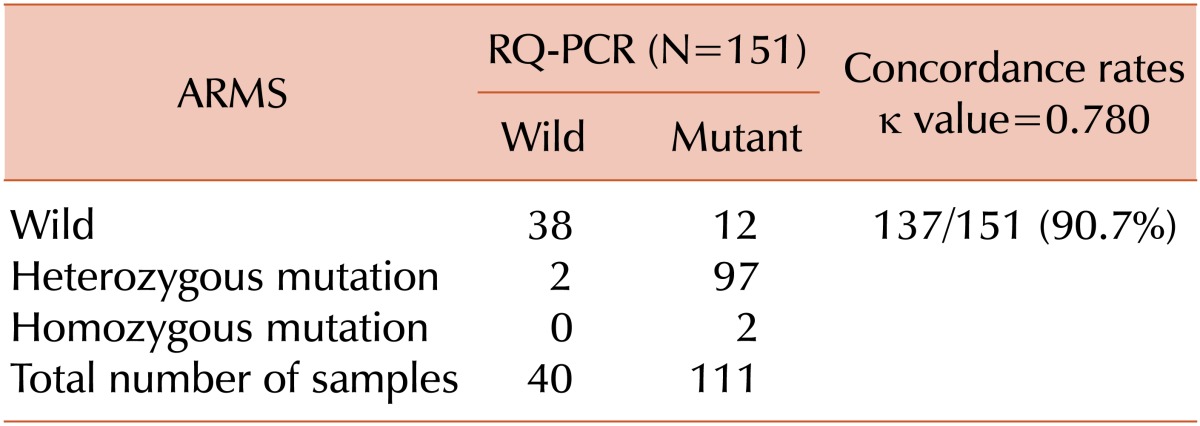
Our data shows that the results of RQ-PCR correspond well with those of allele-specific PCR, with concordance rates of 90.7% and a kappa value of 0.780. The present study showed a low frequency of homozygous positivity in ARMS (only 1 case each of PV and PMF: 1.3%), which may have been caused by the ARMS interpretation method applied at the authors' institution. That is, because the presence of 2 bands was always considered as heterozygous positivity, irrespective of the signal strength; moreover, in this study, the frequency of heterozygous positivity may have been overestimated and that of homozygous positivity may have been underestimated.
Among the cases where the 2 tests showed discrepant results, 12 showed positive RQ-PCR results and negative ARMS results. The majority of these cases were 9 patients with other diseases and a relatively low allele burden of JAK2 V617F (mean, 9.37%), and the other 3 patients were diagnosed with ET and showed a low allele burden of JAK2 V617F (mean, 7.97%). These results suggest that RQ-PCR could be used to detect the JAK2 V617F mutation in 12 samples more than that by the allele-specific PCR and that RQ-PCR may be a more sensitive diagnostic tool to detect the JAK2 V617F mutation than allele-specific PCR methods. The discrepancy between the 2 test results could be attributed to differences in the reaction conditions used in the 2 methods. In our study, 2 µL of template DNA was used for the ARMS test, and it was diluted to a final volume of 25 µL. For RQ-PCR, 5 µL of template DNA was used, and it was diluted to a final volume of 20 µL. Thus, 3.125 times more template DNA was used for RQ-PCR than for ARMS tests. Additionally, 50 amplification cycles were used for RQ-PCR, and 35 cycles were used for ARMS. These differences in reaction conditions would likely result in differences in the detection sensitivity between the 2 methods. For the 2 ET cases that showed negative RQ-PCR results and positive ARMS results, which are very unusual, we did not perform comprehensive analysis to identify the cause, but the discrepancy may have been caused by false-positive ARMS results.
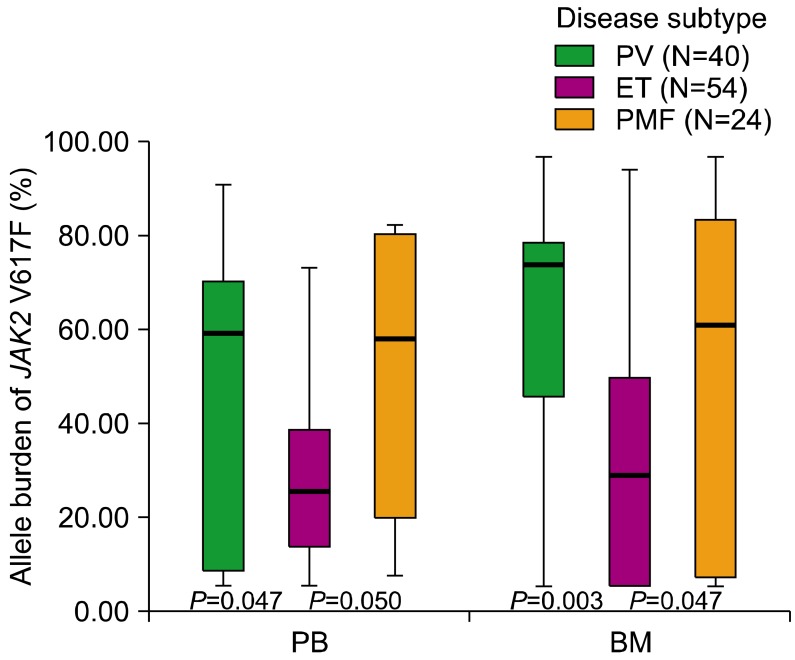
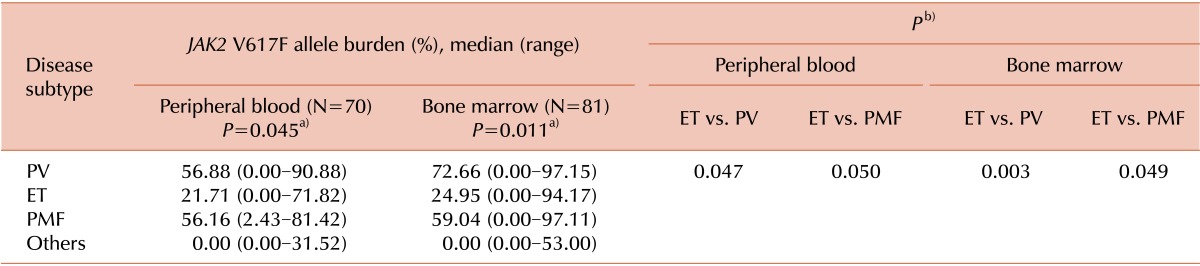
Notably, our results also revealed that the allele burden of
JAK2 V617F differed significantly among the 3 disease subtypes for both PB and BM samples. Our results also showed that the allele burden of
JAK2 V617F was significantly lower for ET than for PV and PMF in both PB and BM samples. These results are consistent with those of previous studies that showed that the allele burden of the
JAK2 V617F mutation was significantly higher in PV than in ET [
6-
8]. These findings can be explained by the fact that approximately one-third of patients with PV and PMF are shown to be homozygous for the
JAK2 V617F mutation. Accordingly, the allele burden of the
JAK2 V617F mutation is considered high in these patients. However, patients with ET are rarely homozygous for the
JAK2 V617F mutation. Consequently, the allele burden of the
JAK2 V617F mutation for patients with ET is expected to be lower than that for patients with PV and PMF [
4-
8]. Our results further support the proposal that the allele burden of the
JAK2 V617F mutation differs substantially among the disease subtypes of Ph
-MPN and that estimation of the allele burden of this mutation can be used as a diagnostic tool to discriminate among the subtypes of Ph
-MPN. We also showed that the allele burden of
JAK2 V617F for ET is significantly lower than that for PV and PMF, regardless of specimen types.
In addition, our results showed that the hemogram results and allele burden of
JAK2 V617F differed significantly among to the disease subtypes based on the higher WBC and hemoglobin counts for PV than for the other subtypes or higher platelet counts for ET than for the other subtypes. However, the relationship between the allele burden of
JAK2 V617F and the hemogram results was not evident. The above results in our study correspond with the results of a recent study, in which no significant correlations were shown between the allele burden of
JAK2 V617F and patient age, WBC, hemoglobin, or the platelet count for PV, ET, or PMF patients [
12].
In conclusion, the allele burden of JAK2 V617F was significantly lower for ET than for PV and PMF, in both PB and BM samples. The quantification of the JAK2 V617F allele burden provides a diagnostic tool for discriminating PV or PMF from ET, regardless of the specimen types.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download