Abstract
Objectives
Desmoplastic melanoma of the oral cavity is an extremely rare condition that is often confused on initial diagnosis with non-melanotic benign lesion or spindle cell tumors. The purpose of this article was to raise awareness of the disease using a literature review.
Materials and Methods
We analyzed 19 desmoplastic melanoma cases reported in the literature and added our experience. Data on clinical, histopathology, treatment, and survival were retrieved and analyzed. Survival analysis was by the Kaplan-Meier method.
Results
Initial clinical and histopathological features were indistinctive, and a definite diagnosis of desmoplastic melanoma at initial assessment was possible in only 23.5% of cases. Among tests, immunohistochemical studies for S-100 and vimentin were all positive. The 5-year disease-free survival rate for oral desmoplastic melanoma was 0%, and the 5-year overall survival rate was 55.0%.
Desmoplastic melanoma is a rare malignancy that was first described by Conley et al.1 as a rare variant of spindle cell melanoma. Melanoma can have various histological patterns depending on its radial and vertical growth phases. Desmoplastic melanoma is usually composed of an inconspicuous superficial melanotic lesion that often lacks pigmentation and a more prominent (and essential) dermal fibrous spindle-shaped cell tumor2. According to Surveillance, Epidemiology, and End Results data, the overall incidence rate of desmoplastic melanoma is 2.0 per million3. Desmoplastic melanoma most commonly occurs in the skin of the head and neck, followed by the extremities and the trunk3.
Desmoplastic melanoma also arises in the oral cavity. However, oral desmoplastic melanoma is extremely rare, and its clinical behavior is largely unknown. Generally, mucosal melanoma of the head and neck region is deemed to have poorer prognosis than its cutaneous counterparts and is staged differently45. Whether this discrepancy is also justified for the desmoplastic subtype remains unclear.
Since an unawareness of oral desmoplastic melanoma seems to predispose misdiagnoses and subsequent inappropriate treatment, we present a comprehensive review that focuses on oral desmoplastic melanoma. We analyzed previously reported cases of oral desmoplastic melanoma and added a case that we encountered.
We conducted a computerized literature search of the Medical Literature Analysis and Retrieval System online database (MEDLINE) and Google Scholar. Search terms used for each website were: “desmoplastic” and “melanoma” and “oral”; “desmoplastic” and “melanoma” and “gingiva”; “desmoplastic” and “melanoma” and “palate”; “desmoplastic” and “melanoma” and “tongue”; “desmoplastic” and “melanoma” and “mouth floor”; “desmoplastic” and “melanoma” and “gums.”
All series and case reports on desmoplastic melanoma of the oral cavity were included in this review. Abstracts were required to be in English with relevant information to be subject for analysis. Relatively abundant lip lesions were excluded because their exposure to ultraviolet light was considered a distinct etiology, and the tendency to involve the mental nerve was characteristic and different from oral desmoplastic melanoma. A total of 19 articles were identified. Three were excluded: 2 involved cases of desmoplastic melanoma that were present only in metastatic lymph nodes, and 1 was in Italian. Finally, 20 cases of oral desmoplastic melanoma were identified, including 1 case that we encountered and others from 16 relevant articles6789101112131415161718192021. Data on clinical features, histopathologic features, diagnostic features, treatment, and prognosis were collected from each study. Due to the retrospective nature of this study, it was granted an exemption in writing by the Institutional Review Board of the National Cancer Center (Goyang, Korea).
Disease-free and overall survival were analyzed using the Kaplan-Meier method. Statistical analyses were performed with the program R using the survival package22.
A 74-year-old woman visited Oral Oncology Clinic at National Cancer Center for a palatal ulceration that had started 2 months previously. On clinical inspection, the ulcerative lesion was 1.8×1.5 cm, located at the left hard palate approaching the midline, and was grossly limited to the mucosa.(Fig. 1) At 17 months before her visit, she was diagnosed with mucinous adenocarcinoma of the left upper lung, which was T4N0M1a. The patient did not have any apparent symptoms of lung cancer, and no specific marker for targeted therapy was available; thus, the oncologist decided to observe the patient without definitive treatment. Her Eastern Cooperative Oncology Group score was assessed to be zero just before surgery for the palatal lesion.
Before her visit to our hospital, the patient underwent a biopsy of the palatal lesion at a local hospital. The resulting diagnosis was squamous cell carcinoma. Since the patient was under close observation of her lung malignancy at our hospital, and the biopsy showed malignancy, the patient decided to have the palatal lesion treated at our hospital. We obtained the biopsy slide and requested a re-examination by our pathologists. The pathologic diagnosis was a spindle cell proliferative lesion, and a borderline or malignant spindle cell tumor was suspected. The tumor was further evaluated by contrast-enhanced computed tomography (CT). On the CT scan, the tumor was indefinite with only suspicious mucosal layer thickening observed at the tumor site. No bone erosion or abnormal cervical lymph node enlargement was observed. A multidisciplinary consultation was conducted to decide the proper treatment. Radiation therapy was considered inappropriate by the radiation oncologist because of the possible side effects for the head and neck region and its unpredictability in terms of the lung cancer prognosis. As the patient's general condition was considered suitable for surgery, surgical resection of the tumor was planned.
The tumor was resected en bloc with the bony hard palate, while preserving the nasal and sinus membranes. Even with a macroscopic 1-cm safety margin, epithelial dysplasia was diagnosed at the anterior and medial margins using frozen biopsy, and further resection was performed intraoperatively. After surgery, the tumor was diagnosed as desmoplastic melanoma, which was 1.5×1.1 cm and 0.3 cm in depth. Moderately increased cellularity and a moderate degree of nuclear pleomorphism were observed in the dermal tumor. The number of mitoses was 12 per 10 high-powered fields.(Fig. 2) The tumor did not involve the bone, and no venous or lymphatic invasion was present. Perineural invasion was not identified. The epithelial dysplasia diagnosed using frozen biopsy was identified as atypical melanocyte involvement on permanent biopsy. S-100 and HMB45 were focally positive using immunohistochemical staining. Vimentin and smooth muscle actin (SMA) staining was also positive. Ki-67 staining was identified in 5% of the cells. Cytokeratin, EMA, p63, desmin, p53, and CD34 were all negative. The pathologic stage was pT3bNx.
The patient was under regular postoperative surveillance with periodic CT scans. At her last visit, which was at 12 months postoperation, no evidence of recurrence was present on a CT scan or on physical examination. An oroantral fistula was present, which was efficiently sealed without discomfort using a new maxillary denture.
Among the 20 patients identified in our literature search6789101112131415161718192021, including ours, the average age at diagnosis was 53.6 years. Although the sixth decade of life was the most frequent age affected, patients aged 20 to 90 years were diagnosed with the disease. A slight male predilection was observed (male:female=3:2). For those with demographic information available, 8 patients were Asian and 4 were Caucasian. Oral desmoplastic melanoma was most often located at the maxillary alveolus or gingiva, followed by the palate. The mandibular gingiva or buccal mucosa was also involved.(Table 1)
The clinical appearance of oral desmoplastic melanoma varied significantly. Pigmentation of the lesion was often absent, which rendered a clinical diagnosis of melanoma unlikely. Among 18 cases with relevant information available, only 4 described pigmentation over the tumor. The shape of oral desmoplastic melanomas ranged from surface irregularity to a sessile mass to a pedunculated mass, adding further complexity. One-third of the cases presented with surface ulceration.
Incisional biopsy was often undiagnostic or misleading in reports of oral desmoplastic melanoma. Misdiagnoses included epulis fibromatosa with atypical proliferation of melanocytes, fibromatosis, 2 spindle cell variants of squamous cell carcinoma, 2 squamous cell carcinomas, atypical lymphoproliferative disorder, malignant melanoma in situ, 2 malignant melanomas, benign spindle cell neoplasia, and a borderline or malignant spindle cell tumor91314151619. We also found a case in which a combination of bland clinical features and associated denture flanges resulted in a clinical diagnosis of fibrous epulis, leading to simple extirpation of the lesion12. Only 4 cases were correctly diagnosed as desmoplastic melanoma on the first investigation7111720.
Microscopically, oral desmoplastic melanomas often had two components, a parenchymal collagenous tumor located at the dermis layer and the overlying mucosa with junctional activity. The dermal components were spindle-shaped cells arranged in fascicles that formed various patterns. These patterns were described as haphazard, fascicular, herringbone, storiform, neural, or epithelioid. Collagenous stroma was more apparent in hypocellular areas. Cellular atypia and nuclear pleomorphism ranged from mild to marked, depending on the case under review and the region of tumor observed. Mitosis measurements ranged from less than 1 per 10 high-powered fields to 12 per 10 high-powered fields. Kurihara et al.9 identified deep vertical invasion by melanocytes from the clusters of junctional activity. However, junctional activity was absent or not prominent in other cases13. Neural invasion or neurotropism was observed in 8 cases out of 11 with relevant descriptions available.
Among 12 cases with relevant records, immunohistochemical studies for S-100 protein showed positive results in all 12. S-100 immunopositivity in atypical spindle cells was either focal or strong918. Kilpatrick et al.13 showed that S-100 immunopositivity was focal in densely collagenized areas, but diffuse in hypercellular areas. Atypical spindle cells were also positive for vimentin in 10 cases. Vimentin immunopositivity was described as extensive, strong, uniform, or diffuse in most cases12131718. HMB45 expression was negative in 7 cases but positive in 3. Even if HBM45 was expressed, its immunopositivity was rare and focal in 2 cases (including ours)18. In 7 studies, antibodies against cytokeratin were used, and all cases tested negative. Four studies tested aberrant p53 expression, and two cases were positive with varying rates of positive cells. Of two p53-positive tumors, 1 was recurrent. Other sporadic immunoreactivity reports that showed negative results included muscle-specific actin, smooth muscle actin, EMA, CD34, CD68, LMWCK, HMWCK, LCA, desmin, caldesmon, calponin, and p63. As dermal tumor cells were amelanotic, Masson-Fontana staining was negative in 2 cases.
Among the 18 cases with relevant descriptions, surgery was the choice of initial treatment in 16. However, surgery was often simple excision guided by the misdiagnoses from biopsies or clinical impressions. Margin involvement was also common because of the confusing benign appearance of oral desmoplastic melanoma. Four patients underwent postoperative radiation therapy with varying results. Definitive or palliative radiation therapy was also attempted in 2 patients, which resulted in a poor or insignificant prognosis. Topical imiquimod was applied to a patient with recurrence, which resulted in a clinical complete response20. However, the tumor recurred in this case.
We found 3 cases with lymph node metastasis at the time of diagnosis. Four patients developed regional recurrence during follow-up. Distant metastases were reported in 5 cases, of which 1 case showed metastasis to the brain via a cranial nerve.
Relevant information was available for 14 cases for disease-free survival analysis. For oral desmoplastic melanoma, the disease-free survival rate was 30.0% at 2 years and 0% at 5 years. The median disease-free survival time was 16.5 months. Of 10 cases, 8 were local recurrences and 4 were regional recurrences. One patient had local and regional recurrences simultaneously. Another patient suffered local recurrence after regional recurrence. Relevant information for overall survival analysis was available for 15 cases. The overall survival rate was 84.8% for 2 years and 55.0% for 5 years. The median overall survival time was 96 months.
With fewer than 30 cases reported in the literature, oral desmoplastic melanoma is extremely rare. While it is impractical to calculate the incidence of oral desmoplastic melanoma, for reference, desmoplastic melanoma is estimated to be less than 1% of all primary melanoma cases. The age-standardized incidence rate of primary oral melanoma is less than 0.01 per 100,000 person-years323.
The etiology of oral desmoplastic melanoma is generally unknown. Ultraviolet radiation has been associated with cutaneous desmoplastic melanoma because most cases occur in sun-exposed areas, especially the head and neck region. However, ultraviolet radiation is unlikely to be the cause of oral desmoplastic melanoma. Prasad et al.15 suggested that aberrant expression of p53 might be related to mucosal desmoplastic melanoma based on their experience of 6 of 7 cases expressing p53. If we consider only oral desmoplastic melanoma, including our case, 2 of 4 oral desmoplastic melanomas tested positive for p53; however, 1 of the positive specimens was a recurrence. Further investigation is required to validate the effect of aberrant p53 expression in oral desmoplastic melanoma.
During the initial assessment of oral desmoplastic melanoma, misdiagnoses were common. Only 4 of 17 initial diagnoses were desmoplastic melanoma in the cases we analyzed. Oral desmoplastic melanomas generally appeared amelanotic. The clinical impression, which can guide proper pathologic diagnosis, rarely included a differential diagnosis of melanoma in cases of oral desmoplastic melanoma. Associated ulceration may also complicate the diagnosis. Usually, the junctional activity of melanocytes at the dermalepidermal junction suggests the melanocytic origin of a dermal tumor. However, when a biopsy specimen is obtained from an ulcerated area, only the dermal spindle cell tumor is present, without any indication of junctional activity. In these circumstances, the melanocytic origin of the tumor cells may not be clear until appropriate immunohistochemical marker studies are performed.
The diagnosis of oral desmoplastic melanoma is based on histopathologic similarities to the cutaneous counterpart. Accordingly, a maxillofacial pathologist unused to cutaneous malignancies may fail to list oral desmoplastic melanoma in the differential diagnosis of spindle cell tumors arising in the oral cavity. Immunohistochemistry markers such as S-100 or vimentin are crucial for diagnosis but are not pathognomonic because other tumors, such as schwannoma, can share a similar immunohistochemical profile. To overcome the challenges leading to misdiagnosis of oral desmoplastic melanoma, primary incisional biopsy should be done carefully. Importantly, incisional biopsy should include the surrounding, normal-looking mucosa, where aberrant junctional activity of melanocytes could reside, especially near an ulcerated lesion.
Several factors are involved in the frequent local recurrence of oral desmoplastic melanomas. The bland clinical appearance tends to lead clinicians to perform simple excision of lesions, especially when incisional biopsy results are indecisive or misleading. Even when malignancy is suspected or a definite diagnosis of desmoplastic melanoma is made, the unique dualistic feature of the tumors, with epithelial and dermal portions, complicates treatment. Junctional activity can reach beyond the dermal tumor to where no clinical abnormality is seen. This feature mandates intraoperative margin evaluation and a wide safety margin during surgery for oral desmoplastic melanomas. Because of the restricted boundaries of the oral cavity, such treatment may not always be possible. Finally, the high propensity of oral desmoplastic melanomas to involve nearby nerves might predispose these tumors to local recurrences15.
Generally, cutaneous melanoma patients show good survival, with a 5-year disease-specific survival rate of 78.8%24. Cutaneous desmoplastic melanoma is similar, with a 5-year disease-specific survival rate of 84.8%3. In contrast, the 5-year survival rate for conventional oral melanoma is 15% with a median survival time of 25 months25. An advanced stage at diagnosis and anatomic restrictions that limit sufficient resection are often given as the reason for poor prognosis. The 5-year survival rate of 55.0% for oral desmoplastic melanoma is between the poor overall survival from oral melanoma and the high overall survival for skin melanoma. The survival rate of oral desmoplastic melanoma is relatively encouraging considering the high recurrence rate, especially with the inadequate primary treatments that were frequently reported. However, the rarity of cases and the restricted follow-up of some cases make it difficult to determine the true prognosis of oral desmoplastic melanoma. The 5-year survival rate of oral amelanotic melanoma was reported to be 34.4% with a median survival of 29 months26. Notably, this report included 2 cases of oral desmoplastic melanoma.
In conclusion, oral desmoplastic melanoma is an extremely rare entity that requires meticulous considerations for proper diagnosis. Clinical findings that more than 70% of desmoplastic melanomas show no black pigmentation often lead to misdiagnosis of melanoma. Awareness of oral desmoplastic melanoma and meticulous inspection including immunohistochemical studies are required to diagnose this rare disease correctly. Tumor behavior seems to differ from both cutaneous desmoplastic melanoma and conventional oral melanoma. Despite the high propensity for local recurrence, oral desmoplastic melanoma appears to be amenable to appropriate wide surgical resection. Further research should aim to validate the current findings and elucidate the etiology of the disease, which might guide proper alternative treatment methods.
Notes
Authors' Contributions: S.W.C. desinged the study. S.K.M. and S.W.C. wrote the manuscript. J.H.J., K.M.A., and J.Y.P. contributed in data collection and analysis. C.W.Y. performed the histological analysis and wrote parts of the manuscript. All authors read and approved the final manuscript.
References
1. Conley J, Lattes R, Orr W. Desmoplastic malignant melanoma (a rare variant of spindle cell melanoma). Cancer. 1971; 28:914–936. PMID: 5286448.

2. Magro CM, Crowson AN, Mihm MC. Unusual variants of malignant melanoma. Mod Pathol. 2006; 19(Suppl 2):S41–S70. PMID: 16446716.

3. Feng Z, Wu X, Chen V, Velie E, Zhang Z. Incidence and survival of desmoplastic melanoma in the United States, 1992-2007. J Cutan Pathol. 2011; 38:616–624. PMID: 21518379.

4. Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC cancer staging manual. 7th ed. New York: Springer;2010.
5. Kim UK, Heo JH, Hwang DS, Kim YD, Shin SH, Kim JR, et al. Clinical study on malignant melanoma in oral cavity. J Korean Assoc Oral Maxillofac Surg. 2008; 34:611–615.
6. Batsakis JG, Bauer R, Regezi JA, Campbell T. Desmoplastic melanoma of the maxillary alveolus. J Oral Surg. 1979; 37:107–109. PMID: 283198.
7. Chen JH, Meng CL, Chao LS, Tu CN, Shyu KW. Desmoplastic amelanotic melanoma of palate: a case report with immunohistochemistry and electron microscopic studies. Zhonghua Ya Yi Xue Hui Za Zhi. 1989; 8:80–89. PMID: 2484264.
8. Jain S, Allen PW. Desmoplastic malignant melanoma and its variants. A study of 45 cases. Am J Surg Pathol. 1989; 13:358–373. PMID: 2712188.
9. Kurihara K, Sanada E, Yasuda S, Yamasaki H. Desmoplastic malignant melanoma of the gingiva. Oral Surg Oral Med Oral Pathol. 1992; 74:201–205. PMID: 1508529.

10. Devaraj VS, Moss AL, Briggs JC. Desmoplastic melanoma: a clinico-pathological review. Br J Plast Surg. 1992; 45:595–598. PMID: 1493532.

11. Manganaro AM, Hammond HL, Dalton MJ, Williams TP. Oral melanoma: case reports and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995; 80:670–676. PMID: 8680974.
12. Ueta E, Miki T, Osaki T, Iwata J, Sonobe H. Desmoplastic malignant melanoma of the gingiva: case report and review of the literature. Eur J Cancer B Oral Oncol. 1996; 32B:423–427. PMID: 9039229.

13. Kilpatrick SE, White WL, Browne JD. Desmoplastic malignant melanoma of the oral mucosa. An underrecognized diagnostic pitfall. Cancer. 1996; 78:383–389. PMID: 8697380.

14. Kavanagh BD, Campbell RL, Patterson JW, O'Neill RL, Cardinale RM, Kaugars GE. Desmoplastic malignant melanoma of the palatal alveolar mucosa: sustained disease-free survival after surgery and postoperative radiotherapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000; 89:465–470. PMID: 10760728.

15. Prasad ML, Patel SG, Busam KJ. Primary mucosal desmoplastic melanoma of the head and neck. Head Neck. 2004; 26:373–377. PMID: 15054741.

16. Choi G, Kim JW, Nam SY, Cho KJ. Primary mucosal desmoplastic melanoma of gingiva: a case report. Korean J Pathol. 2006; 40:456–460.
17. Ramani P, Chandrasekar T, Narayanan V, Anuja N, Karthikeyan R, Reddy S, et al. Desmoplastic malignant melanoma of alveolus: a rare entity. Oral Oncol Extra. 2006; 42:291–294.
18. Köseoğlu RD, Aladağ İ, Sezer E, Özkan N. Primary mucosal desmoplastic melanoma of the gingiva. Turk J Med Sci. 2009; 39:483–490.
19. Jou A, Miranda FV, Oliveira MG, Martins MD, Rados PV, Filho MS. Oral desmoplastic melanoma mimicking inflammatory hyperplasia. Gerodontology. 2012; 29:e1163–e1167. PMID: 22612831.

20. Smyth EC, Flavin M, Pulitzer MP, Gardner GJ, Costantino PD, Chi DS, et al. Treatment of locally recurrent mucosal melanoma with topical imiquimod. J Clin Oncol. 2011; 29:e809–e811. PMID: 22010009.

21. Belgaumi UI, Shetty P, Shirlal S. Oral malignant melanoma: a case report of an unusual clinical and histologic presentation. Dent Res J (Isfahan). 2013; 10:404–407. PMID: 24019813.
22. R: a language and environment for statistical computing [Internet]. Vienna: R Foundation for Statistical Computing;Available from: http://www.R-project.org/.
23. Sortino-Rachou AM, Cancela Mde C, Voti L, Curado MP. Primary oral melanoma: population-based incidence. Oral Oncol. 2009; 45:254–258. PMID: 18675580.

24. Chang AE, Karnell LH, Menck HR. The National Cancer Data Base report on cutaneous and noncutaneous melanoma: a summary of 84,836 cases from the past decade. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer. 1998; 83:1664–1678. PMID: 9781962.
25. Hicks MJ, Flaitz CM. Oral mucosal melanoma: epidemiology and pathobiology. Oral Oncol. 2000; 36:152–169. PMID: 10745167.

26. Paulo LF, Servato JP, Rosa RR, Oliveira MT, Faria PR, Silva SJ, et al. Primary amelanotic mucosal melanoma of the oronasal region: report of two new cases and literature review. Oral Maxillofac Surg. 2015; 19:333–339. PMID: 25934245.

Fig. 1
Oral desmoplastic melanoma presented as an ulcerative lesion of the left hard palate. The tumor was 1.8×1.5 cm.
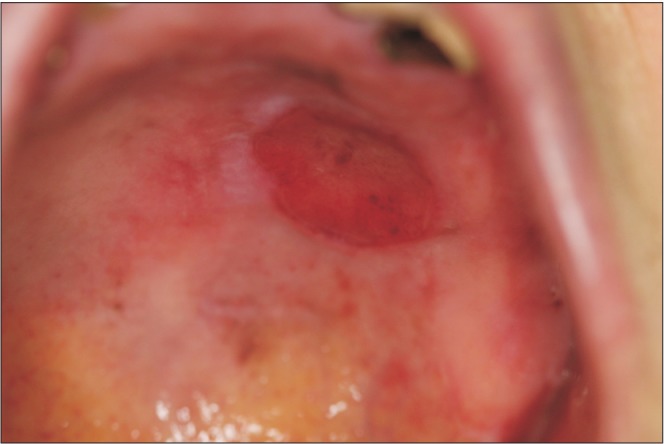
Fig. 2
Histopathologic appearance of desmoplastic melanoma. A. Spindle-shaped tumor cells of the submucosa with normal-looking squamous epithelium (H&E staining, ×40). B. Junctional activity at the dermoepithelial junction (H&E staining, ×100). C. Submucosal tumor cells with haphazard, fascicular growth pattern with intermixed collagenous stroma. No definite melanin pigmentation is noted (H&E staining, ×100). D. Bland-looking tumor cells with minimal pleomorphism or atypia (H&E staining, ×200).
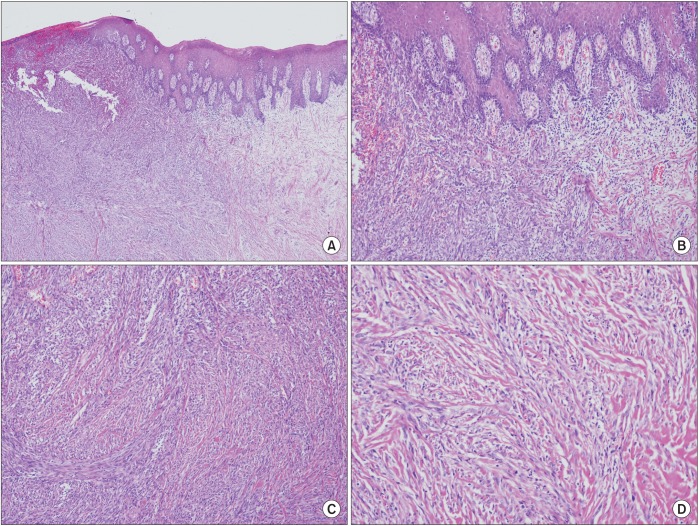
Table 1
Summary of clinical features of reported cases
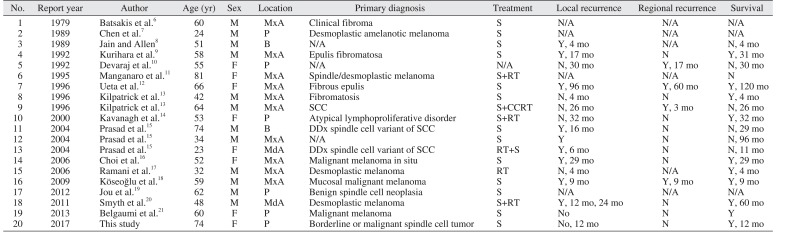
| No. | Report year | Author | Age (yr) | Sex | Location | Primary diagnosis | Treatment | Local recurrence | Regional recurrence | Survival |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1979 | Batsakis et al.6 | 60 | M | MxA | Clinical fibroma | S | N/A | N/A | N/A |
| 2 | 1989 | Chen et al.7 | 24 | M | P | Desmoplastic amelanotic melanoma | S | N/A | N/A | N/A |
| 3 | 1989 | Jain and Allen8 | 51 | M | B | N/A | S | Y, 4 mo | N/A | N, 4 mo |
| 4 | 1992 | Kurihara et al.9 | 58 | M | MxA | Epulis fibromatosa | S | Y, 17 mo | N | Y, 31 mo |
| 5 | 1992 | Devaraj et al.10 | 55 | F | P | N/A | N/A | N, 30 mo | Y, 17 mo | N, 30 mo |
| 6 | 1995 | Manganaro et al.11 | 81 | F | MxA | Spindle/desmoplastic melanoma | S+RT | N/A | N/A | N |
| 7 | 1996 | Ueta et al.12 | 66 | F | MxA | Fibrous epulis | S | Y, 96 mo | Y, 60 mo | Y, 120 mo |
| 8 | 1996 | Kilpatrick et al.13 | 42 | M | MxA | Fibromatosis | S | N, 4 mo | N | Y, 4 mo |
| 9 | 1996 | Kilpatrick et al.13 | 64 | M | MxA | SCC | S+CCRT | N, 26 mo | Y, 3 mo | N, 26 mo |
| 10 | 2000 | Kavanagh et al.14 | 53 | F | P | Atypical lymphoproliferative disorder | S+RT | N, 32 mo | N | Y, 32 mo |
| 11 | 2004 | Prasad et al.15 | 74 | M | B | DDx spindle cell variant of SCC | S | Y, 16 mo | N | N, 29 mo |
| 12 | 2004 | Prasad et al.15 | 34 | M | MxA | N/A | S | Y | N | N, 96 mo |
| 13 | 2004 | Prasad et al.15 | 23 | F | MdA | DDx spindle cell variant of SCC | RT+S | Y, 6 mo | N | N, 11 mo |
| 14 | 2006 | Choi et al.16 | 52 | F | MxA | Malignant melanoma in situ | S | Y, 29 mo | N | Y, 29 mo |
| 15 | 2006 | Ramani et al.17 | 32 | M | MxA | Desmoplastic melanoma | RT | N, 4 mo | N/A | Y, 4 mo |
| 16 | 2009 | Köseoğlu et al.18 | 59 | M | MxA | Mucosal malignant melanoma | S | Y, 9 mo | Y, 9 mo | Y, 9 mo |
| 17 | 2012 | Jou et al.19 | 62 | M | P | Benign spindle cell neoplasia | S | N/A | N/A | N/A |
| 18 | 2011 | Smyth et al.20 | 48 | M | MdA | Desmoplastic melanoma | S+RT | Y, 12 mo, 24 mo | N | Y, 60 mo |
| 19 | 2013 | Belgaumi et al.21 | 60 | F | P | Malignant melanoma | S | No | N | Y |
| 20 | 2017 | This study | 74 | F | P | Borderline or malignant spindle cell tumor | S | No, 12 mo | N | Y, 12 mo |




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download