Abstract
Temporomandibular disorders (TMDs) are diseases that affect the temporomandibular joint and supporting structures. The goal of treatment for TMDs is elimination or reduction of pain and return to normal temporomandibular joint function. Initial treatment for TMDs is non-invasive and conservative, not surgical. Oral and maxillofacial surgeons should fully understand and actively care about non-invasive treatments for TMDs. The purpose of this study is to review the validity and outcomes of non-invasive and surgical treatment modalities for TMDs.
Temporomandibular disorders (TMDs) are diseases that affect the temporomandibular joint (TMJ) and supporting structures1. The goal of treatment for TMDs is elimination or reduction of pain and joint sounds and a return to normal TMJ function. Such treatment involves a diet of soft foods, behavior modification, pharmacotherapy, inter-occlusal splints, intra-articular injections, physical therapy, arthrocentesis, arthroscopy, or open joint surgery. In recent years, low-level laser therapy (LLLT) has been introduced as a non-invasive physical treatment for TMDs and myofascial pain23. Because the pathogenic pathways cannot be clearly defined, reversible treatment, minimally invasive treatment, or no treatment is generally preferred. If conservative treatment fails, surgical treatment is then considered. The purpose of this article is to review the mechanisms, outcomes, and protocols of the main treatment methods for TMDs: pharmacotherapy, physical therapy, inter-occlusal splints, hyaluronic acid (HA) joint injection, botulinum toxin (BTX) type A injection, arthrocentesis, and arthroscopic treatment.
Repetitive loading and macro- or microtrauma from masticatory muscles cause microlesions at masticatory muscle fibers, resulting in the release of local inflammatory mediators, such as prostaglandins, bradykinin, histamine, and substance P. These mediators can induce nociceptive afferent impulse transmission to the superior nervous center, causing peripheral and central sensitization4. Pain relief through the use of non-steroidal anti-inflammatory drugs (NSAIDs) might result from reduction of peripheral sensitization by decreasing the fatigue, overload, and release of inflammatory components in masticatory muscles and the TMJ5. There are different types of NSAIDs, such as ibuprofen (average daily dose of 400–800 mg), diclofenac sodium, and meloxicam (average daily dose of 7.5–15 mg). NSAIDs can eliminate pain and also block inflammation-induced upregulation of sodium channel 1.7 (Nav1.7) in the trigeminal ganglion (TG). TMJ inflammation increases the excitability of neurons in the TG, and NAv1.7 plays an important role in inflammatory pain. Moreover, NSAIDs are known to reduce inflammation through inhibition of cyclooxygenase-1 and -2 (COX-1 and COX-2); COX-2 is important in prostaglandin (PGE2) synthesis, which is related to joint pain6. Studies have compared the treatment potential of glucosamine sulfate (GS; an amino sugar and a precursor in the biomechanical synthesis of glycosylated proteins and lipids) and ibuprofen in patients diagnosed with TMJ osteoarthritis (OA). In a previous study, 40 women and 5 men in a randomized double-blind study received either GS (500 mg three times a day [t.i.d.]) or ibuprofen (400 mg t.i.d.) for 90 days. TMJ pain with function, absence of pain, and voluntary maximum mouth opening were assessed. The Brief Pain Inventory questionnaire and a test for masticatory muscle tenderness were administered after a one week washout period and at Day 90. Fifteen GS patients (71%) and 11 ibuprofen patients (61%) improved, with a positive clinical response defined as a 20% decrease in the primary outcome (TMJ pain with function). The number of patients with a positive clinical response did not differ statistically between the groups (P=0.73). Between-group comparisons revealed that the patients taking GS had a significantly greater decrease in TMJ pain with function and effect from pain compared with patients taking ibuprofen. The most commonly used NSAIDs are selective COX-2 NSAIDs because of their gentle effect on the gastric lining; examples of such drugs are meloxicam7 and celecoxib (Celebrex). The dose range for celecoxib is 100 to 200 mg up to twice a day, with lower doses prescribed for OA and higher doses used for pain and rheumatoid arthritis8. However, that celecoxib dose provided no statistically significant reduction in pain in a randomized, double-blind, placebo-controlled trial (n=68)9. The rofecoxib dose range is 12.5, 25, or 50 mg/day, with a lower dose for OA and higher doses for pain and rheumatoid arthritis10. Previously, 600 mg of ibuprofen four times per day provided no statistically significant reduction in pain, but a combination of ibuprofen and diazepam was more effective than placebo in a double-blind randomized controlled trial (n=39)11.
Bruxism and TMDs are commonly associated with depression12. Bruxism results from an imbalance between the direct and indirect pathways of the basal ganglia through the disruption of action potential transmission, which is mediated by dopamine. Dopamine agonists are therefore helpful in treating bruxism because they increase dopamine levels and restore the balance between the basal ganglia pathways. For example, selective serotonin reuptake inhibitors such as fluoxetine, sertraline, and paroxetine increase concentrations of serotonin. Serotonin also suppresses dopamine release from the neocortical tract, where dopamine prevents spontaneous movements13. Another type of dopamine agonist is serotonin-norepinephrine reuptake inhibitors such as venlafaxine, which block both serotonin and norepinephrine reuptake14. The tricyclic antidepressants (TCAs) have analgesic properties, so they are a good choice for treating depressed patients who also suffer from a TMD, especially with chronic pain. Amitriptyline, a TCA, can offer pain relief at a dose of 75 mg, but increasing the dose can cause dry mouth and drowsiness; it also carries the serious side effect of cardiotoxicity. In a double-blind randomized clinical trial, 25 mg of amitriptyline per day provided a statistically significant reduction in pain (n=12)1516. The TCAs amitriptyline, nortriptyline, and desipramine are frequently prescribed for TMD pain. These drugs also block the reuptake of catecholamines and might therefore increase catecholaminergic neurotransmission. Thus, the administration of an exogenous catecholamine such as epinephrine as a local dental anesthetic could cause adverse cardiovascular events; the amount of epinephrine should be limited to 0.04 mg per appointment for patients taking TCAs17.
Imotun is an avocado-soybean unsaponifiable extract (ASU) that appears to have anabolic, anti-catabolic, and anti-inflammatory effects on chondrocytes. It protects cartilage by inhibiting the effects of interleukin 1 and collagenase, and it reduces inflammation by inhibiting prostaglandin E2, cyclooxygenase A2, and nitric oxide. It also stimulates chondrocytes and synoviocytes through proteoglycan and synovial fluid synthesis. At the clinical level, ASU reduces pain and stiffness while improving joint function1819. The administration of ASU in a single daily dose (300 mg) might contribute to the absence of side effects, but the literature reports no differences between the use of one or two daily doses (600 mg).
LLLT works through photobiomodulation20, which is based on metabolic activation through stimulation of the cellular respiratory chain in mitochondria that in turn increases vascularization and enhances the supply of oxygen in hypoxic cells. At the cellular level, LLLT releases protons into the cytoplasm, which reduces the permeability of the duct to sodium (Na+) and potassium (K+) ions and decreases the action potential frequency. Although some researchers have attributed the analgesic and anti-inflammatory properties of LLLT to an increase in the production of β-endorphin, reductions in the secretion of histamine and acetylcholine and the synthesis of bradykinin, as well as an increase in the production of adenosine triphosphate, can result in muscle relaxation and an increase in blood microcirculation, thereby accelerating the clearance of catabolites from tissues21. The treatment was performed twice a week for three consecutive weeks with a 780 nm GA-Al-As (gallium-aluminum-arsenide) diode laser (twin laser). Given uncertainty about which laser dose would be the most effective, the researchers used the protocol suggested by the Department of Optical and Photonic Physics at the University of São Paulo State (USP), São Carlos, São Paulo, Brazil. The experimental group received LLLT with an output of 30 mW, a time of 10 seconds, and 6.3 J/cm2 at three points in each TMJ, (1) the posterior aspect of the joint with the mouth open to treat the posterior articular branches of the auricular-temporal nerve; (2) the area anterior to the condyle in the sigmoid notch with the mouth closed, which gives the patient a short rest and is the area of insertion of the lateral pterygoid muscle into the condylar neck and disc; (3) the joint interface with the mouth open22. Muscles with painful symptoms should be treated with the laser beam applied point wise with an ED of 3 J/cm2 at three predetermined points on the masseter and temporal muscles2324. Pinheiro et al.25 used LLLT with wavelengths of 632.8, 670, and 830 nm and an average dose of 1.8 J/cm2 to treat 24 TMD patients. They found significant recovery from pain and clicking in the treated patients. Using a GA-As laser at 904 nm and a light dose of 3 J/cm2, Kulekcioglu et al.23 treated 35 TMD patients and found a significant reduction in pain.
Therapeutic ultrasound has an output of 20 to 60 kHz. It produces deep heat at joints and treats joint contracture by increasing the stretch of the extra capsular soft tissue. It also decreases non-acute pain, muscle spasms, and tendonitis, facilitating the stretch of soft tissue by decreasing the viscosity of collagen, thereby decreasing the firing of type II muscle spindles and facilitating the breakage of calcium deposits in bursitis26. Electrical stimulation devices for the treatment of TMDs have two main purposes: relief of pain and relief of muscle hyperactivity or spasm. Such devices use one of two electrical modalities: transcutaneous electrical nerve stimulation (TENS), which applies a low-voltage, low-amperage, biphasic current at varying frequencies, and high-voltage galvanic stimulation, which applies a higher (>150 V) voltage, low-amperage, monophasic current at varying frequencies27. Sonography has been used to measure masseter muscle thickness28. Ariji et al.29 investigated the sonographic features of inflammatory myopathies with reference to histologic findings and confirmed that the thickness of edematous muscle was greater than that of non-edematous muscle. Although the cause of thickening is not well understood, two possible causes have been suggested. When a muscle contracts, the muscle fiber filament slides and increases the fiber diameter. Another cause could be an edematous change in the muscle. El Fatih et al.30 reported that the ultrasound group showed a higher success rate (93.3% pain improvement) than the TENS group (success rate of 53.3%). Esposito et al.31 concluded that ultrasound is most successful in alleviating muscle symptoms and less effective in reducing symptoms associated with the disc. Masseter muscle thickness significantly decreased after treatment on the symptomatic side in both groups, with no significant differences found between the modalities of treatment (P>0.05). The decrease in thickness suggests that the massage treatment of the ultrasound improved the imbalance in the masticatory muscles. The TENS electrodes can be made of silicone and are applied with gel placed between them and the skin, or they can be self-adhesive. They are positioned at the origin of pain or as close as possible to the highest pain site, within the same dermatome and myotome, and on myofascial trigger points or acupuncture points. There is also the option of placing them on the pathway of peripheral nerves involved with pain genesis. TENS uses different pulse frequencies (high frequency, higher than 50 Hz, and low frequency, lower than 10 Hz), intensities, and durations. TENS used in dentistry generally uses a mix of high and low frequencies. Studies indicate that intensities varying between 10 and 30 mÅ are the most adequate, producing few fasciculations. Values from 40 to 75 µs are recommended for the pulse time32.
Occlusal splints are removable artificial occlusal surfaces that affect the relationship of the mandible to the maxilla and are used for diagnosis or therapy333435. The mechanisms of action as a treatment involve restoring the vertical dimension of occlusion, occlusal disengagement, joint unloading, masticatory muscle relaxation, or TMJ repositioning. Occlusal appliances are fabricated of either hard or soft acrylic resin. Hard acrylic resin occlusal appliances have several advantages over the soft appliances: the hardness of the resin enables quick and easy adjustments and easy repairs; the fit of the hard acrylic resin is more accurate, and methods of fabrication are more reliable; the hard resin also offers greater longevity, more color stability, less food debris accumulation, and more durability than the soft resin. The Acheson classification of occlusal appliances includes muscle relaxation appliances or stabilization appliances used to reduce muscle activity; anterior repositioning and orthopedic repositioning appliances; anterior bite plane and pivoting appliances; and soft or resilient appliances. The flat plane stabilization appliance (Michigan splint) is fabricated for the maxillary arch, but because it interferes with speech and aesthetics, it is often placed on the mandibular arch instead. This splint is supposed to protect teeth, relax the elevator muscles, provide stabilization, redistribute occlusal forces, and inhibit bruxism. The stabilization splint (SS), also known as the Tanner appliance, Fox appliance, Michigan splint, or the centric relation appliance, is a hard acrylic splint that provides temporary and removable ideal occlusion (ideal contact between the teeth for the muscles and the TMJs). Providing ideal occlusion through splint therapy reduces abnormal muscle activity and produces neuromuscular balance. Normally, patients wear the splint only at night. Over several visits, the splint needs to be adjusted by grinding some of its surface points because the lower jaw will adopt a new position as a result of wearing the splint. Eventually, the masticatory muscles relax into a consistent jaw relationship. The patients should then be reviewed at regular intervals. After a period of successful splint therapy (normally between two to three months), patients can be weaned off the splint. SS therapy can be beneficial for reducing depression and pain severity at rest and on palpation compared to no treatment24. In a 2004 Cochrane Review, Al-Ani et al.36 found insufficient evidence to support the use of splint therapy for the treatment of TMDs. Qvintus et al.37 further evaluated the long-term effects of splint therapy; after 1 year, 27.6% of TMD patients who received splint treatment and 37.5% of TMD patients who received counseling and instructions for masticatory muscle exercises reported ‘very good’ treatment effects.
OA is a common TMD. Patients with this disorder tend to exhibit a reduction in intra-articular HA concentration due to depolymerization by reactive oxygen species and production of acid molecules with a lower molecular weight than normal38. Consequently, lubrication is reduced, and joint mechanical stress is increased, resulting in clinical and radiographic progression of the disease. HA provides a longterm lubricating effect, reducing the actions of inflammatory mediators and increasing joint mobility. Results from studies with an active control group suggest that arthrocentesis plus HA injection is superior to arthrocentesis alone in patients with closed lock39. A protocol of two HA injections at weekly intervals is more effective than placebo injections of saline23, and joint catabolite levels decreased significantly with a supplemental HA injection4041. All studies reported a decrease in pain levels; other positive outcomes considered were condylar mobility42, kinesiographic43 and electromyographic recordings44, and a reduction in the Helkimo clinical dysfunction index40. Range of motion improved in the majority of studies, but a couple of papers4546 reported the absence of significant changes in mouth opening.
BTX is a strong biological exotoxin produced by Clostridium botulinum , a Gram-positive anaerobic bacterium. There are seven types of BTX, specified by the letters A to G, among which the strongest organic toxin is BTX-A. The mechanism of action is related to blocking the release of acetylcholine from a presynaptic neuromuscular synapse and, in the autonomous nervous system, blocking its release from postganglionic cholinergic neurons. BTX-A is used to treat bruxism, myofascial pain, disorders associated with TMJ disc displacement, and habitual dislocation of the mandible. The neurotoxin paralyzes the skeletal muscles by blocking calcium-dependent acetylcholine from nerve endings, causing functional denervation. The local paralysis is reversible; neuron regrowth occurs after 2 to 4 months4748. In experimental studies4950, a dose of 12.5 to 200 units per muscle was used. In three experimental studies495051, a single injection was administered; in another study52, 17% of patients received a second injection. Generally in these studies, the effects of the single dose lasted until the end of the follow-up period. In all investigated cases, the first dose yielded relatively favorable outcomes, and doses repeated after 2 to 6 months were performed to strengthen the effect of the previous injection. Treatment with BTX is contraindicated in women during pregnancy and lactation, in patients with known hypersensitivity to any component of the drug (for example, human albumin), during an infection or inflammation of the area to be injected, and in patients treated with aminoglycoside antibiotics, cyclosporine, D-penicillamine, tuba curare, succinylcholine, chloroquine, or hydroxyl chloroquine. BTX has immunogenic properties that can lead to the stimulation of antibody production. Antibodies can be either neutralizing (interfere with the activity of the drug and reduce the effectiveness of the treatment) or non-neutralizing (do not affect the drug) and are formed in response to repeated exposure to the neurotoxin protein complex. To minimize resistance to the BTX resulting from the production of autoantibodies, the lowest effective dose should be applied, with at least three-month intervals between treatments. Booster injections are not recommended. To treat masticatory muscle disorders, the injections are performed with a 12 mm needle and a force of 30 g, strictly intramuscularly after careful aspiration, and the muscle must be relaxed50. While the neurotoxin is being injected, the patient should sit in the dental chair; after the injection, the patient must remain in an upright position for four hours to reduce diffusion to the throat muscles, which carries risk of reflux53. BTX injections reduced the maxillofacial muscle pain associated with TMJ dysfunction52. Electromyography was used during injection in muscles that were difficult to access, including the lateral pterygoid muscle (8 out of 41 patients). Accordingly, localized pain occurred in 80% of patients and remained in about half of the patients for 3 months after injection52. Researchers noted adverse effects such as muscle paralysis and dysphagia in a number of patients54 but described those effects as temporary. They also pointed out that, four weeks after beginning the treatment, about 91% of patients reported improvement in facial pain54. Song et al.55 investigated the effects of BTX on the treatment of TMDs. They collected and defined an algorithm for treating TMD patients and determined that conservative treatments such as warm compresses, behavioral therapy, oral appliances, and drugs (anti-inflammatory drugs and muscle relaxants) should be used prior to BTX-A injection55. Emara et al.49 assessed the effects of BTX in the lateral pterygoid muscle for the treatment of joint click in 6 patients (11 joints). They observed that toxin injection eliminated the click sound in 10 joints during the first week and in one joint after one week. During the following three to four months, recurrence of the click sound was reported in only one joint49. A recent systematic review of BTX-A in the treatment of TMDs included five relevant trials involving 117 participants. Two trials revealed a significant between-group difference in myofascial pain reduction. A trial that compared BTX with fascial manipulation showed equal efficacy of pain relief from TMDs, and the other two trials showed no significant difference between the BTX and placebo groups.
Arthrocentesis occurs in three steps: forcing apart the joint constituents, washing away inflammation and degradable products, and eliminating intra-articular effusion to decrease intra-articular pressure, control pain, and improve function. High success rates have been reported for internal derangements (ID) and closed lock56. If arthrocentesis does not bring pain relief, the patient has a pathologic condition unresponsive to lavage, such as fibrous adhesions or osteophytes, which requires surgical correction. The effectiveness of arthrocentesis is temporary; also, it does not rehabilitate the micro-architecture of the TMJ. Injecting some biological or non-biological agent with tissue regeneration capacity into the TMJ might help stimulate regeneration processes, especially because intra-articular injections can stimulate regeneration and inhibit degenerative changes in the cartilage57. Different intra-articular injection agents, such as HA, corticosteroids, diclofenac sodium, and platelet-rich plasma, have been used to treat TMJ OA. The puncture site is performed 10 mm away and 2 mm below the canthus tragus line. The syringe has to be directed in a 45° angle from posterior to anterior and from downward to upward until the edge of the temporalis fossa is about 15 mm from the skin. An anterior puncture 20 mm away and 7 mm below the canthus tragus line is used for drainage. Instillation of 250 to 300 cm3 of Ringer solution is recommended for the entire procedure.
Surgical arthroscopy was introduced for anterior release of the joint capsule or the pterygoid muscle to allow for posterior repositioning of the disc and electrocautery of the posterior ligament. Good results were obtained by Davis et al.58. For the single puncture technique, the patient is prepared and draped in the standard fashion for arthroscopy. Approximately 2 mL of lactated Ringer solution is injected into the superior joint space with a 22-gauge needle. Next, an incision measuring approximately 0.5 cm is made along the pre-tragal skin crease. A 1.9-mm sharp trocar with a cannula is then inserted using a standard inferolateral technique. Once inside the joint, the sharp trocar is removed, and a 30° arthroscope is inserted. Next, an 18-gauge needle is placed as an outflow tract. Diagnostic arthroscopy is then performed in a standard fashion. Once the examination is complete, the arthroscope is removed. While the cannula is maintained in place, a switch stick is inserted to allow removal of the standard cannula and its replacement with a cannula with a laser portal (400 µm). Once the specialized cannula is inserted, a laser fiber (200 µm) can be inserted through the portal, and surgical maneuvers can be performed with a holmium:YAG laser. It is important to back the laser fiber slightly out of the joint while manipulating the cannula to a new position to avoid breaking the tip of the laser fiber. Once all the procedures have been performed, the laser fiber is removed, and arthrocentesis is performed with 400 mL of lactated Ringer solution. The joint space is injected with a Kenalog (Bristol-Myers Squibb, New York, NY, USA)/0.5% plain Marcaine (Hospira, Lake Forest, IL, USA) mixture, followed by hyaluronan. The incision is then closed with the single-cannula technique introduced by Stringer and Park59 for operative arthroscopy. This technique allows visualization of the TMJ, but once the examination is complete, the endoscope is removed, and the laser is inserted through the cannula, leaving the surgeon to perform the surgical maneuvers without visualization.
The OSCA technique provides the ability to perform three operations through one system: one-track arthrocentesis, standard arthrocentesis with diagnostic visualization, and visually guided surgery. This technique is relatively simple and includes a single working cannula without the need for a second puncture. Moreover, OSCA seems to be as effective as the traditional TMJ arthroscopy technique in regard to pain relief and mouth opening improvements. In addition, operative time is quite short, especially as the surgeon becomes familiar with the technique. The 55% decrease in pain level and 34% increase in maximum mouth opening following the OSCA procedure are within the ranges reported for standard techniques6061. Arthroscopic lysis and lavage is a standardized procedure for the treatment of chronic closed lock of the TMJ. The effect of arthroscopic lysis and lavage lies mostly in the irrigation of the joint cavity, washout of inflammatory cytokines, mobilization of the articular disc, stretching of the capsule, and lysis of intra-articular adhesions62. Machoň et al.63 evaluated the efficacy of arthroscopic lysis and lavage in treating patients with chronic closed lock and found that patients with a shorter duration of symptoms benefited more than those with a longer duration. Indresano62 hypothesized that lysis and lavage can reverse the problem when it is caught early, and that operative arthroscopy is required for prolonged, advanced stages of the disease. Patients in the early to intermediate stage had excellent results in 94% of cases. Smolka and Iizuka64 and Smolka et al.65 found similar results. Complications after arthroscopy of the TMJ usually present in the immediate postoperative phase and are mostly associated with fluid collection and vascular injury. Other rare complications include Horner syndrome, extravasation of fluid from the articular capsule, upper airway compression, variant petrotympanic fissure, infection of the infratemporal space, pseudoaneurysm, and arteriovenous fistula. Arthroscopic lysis and lavage was found to be effective in increasing mouth opening in patients suffering from intermediate/late stage ID and in decreasing and even eliminating the intermittent locking episodes and transient pain that characterize patients with early/intermediate stage ID. Several studies demonstrating the efficacy of TMJ arthroscopy have been published. In a multicenter study by McCain et al.66 involving 6 years and 4,831 joints, patients responded very well to TMJ arthroscopy for various forms of ID. TMJ arthroscopic lysis and lavage was specifically successful in intermediate and intermediate/late progression of ID (Wilkes stages III and IV), as reported by Murakami et al.67, who showed an average 90% success rate with arthroscopic lysis and lavage in a 5-year follow-up study.
Patient examination and discussion and magnetic resonance imaging (because most cases involve disc displacement) are followed by impression collection for splint construction (mostly maxillary SSs) to regain a nonpainful maxillomandibular relationship. Adjunctive therapies to splint therapy are LLLT and medications (such as CH Alpha, a liquid containing bioactive collagen peptides, and a patented compound called Fortigel).
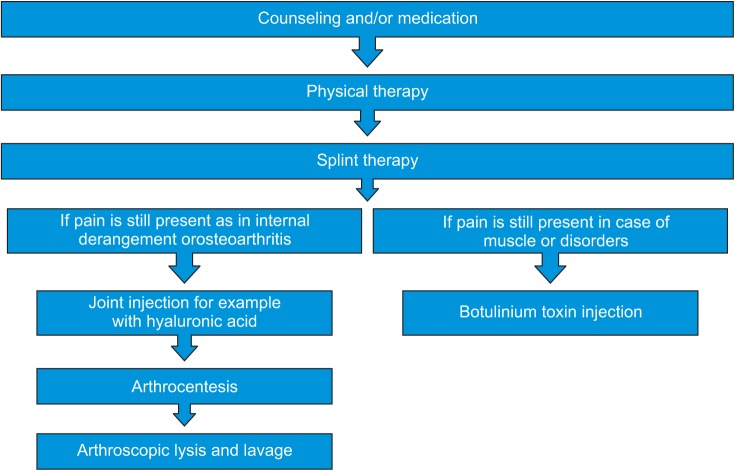
Treatments range from simple self-care practices and conservative treatments to injections and open surgery.(Fig. 1) Most experts agree that treatment should begin with conservative, nonsurgical therapies, with surgery left as the last resort. Many of the treatments discussed here work best when used in combination.
Notes
Authors' Contributions: A.M.A. participated in literature review and wrote the manuscript. A.K.K. and S.A.H. participated in the study design and performed the literature collection. Y.K.K. participated in the study design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
References
1. Carlson CR, Okeson JP, Falace DA, Nitz AJ, Curran SL, Anderson D. Comparison of psychologic and physiologic functioning between patients with masticatory muscle pain and matched controls. J Orofac Pain. 1993; 7:15–22. PMID: 8467294.
2. Wang X, Yang Z, Zhang W, Yi X, Liang C, Li X. Efficacy evaluation of low-level laser therapy on temporomandibular disorder. Hua Xi Kou Qiang Yi Xue Za Zhi. 2011; 29:393–395. PMID: 21932661.
3. Sipahi A, Satilmis T, Basa S. Comparative study in patients with symptomatic internal derangements of the temporomandibular joint: analgesic outcomes of arthrocentesis with or without intraarticular morphine and tramadol. Br J Oral Maxillofac Surg. 2015; 53:316–320. PMID: 25623934.

4. Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci. 2009; 32:1–32. PMID: 19400724.

5. Cairns BE, Mann MK, Mok E, Dong XD, Svensson P. Diclofenac exerts local anesthetic-like actions on rat masseter muscle afferent fibers. Brain Res. 2008; 1194:56–64. PMID: 18199427.

6. Dina OA, Green PG, Levine JD. Role of interleukin-6 in chronic muscle hyperalgesic priming. Neuroscience. 2008; 152:521–525. PMID: 18280048.

7. Simon LS, Lanza FL, Lipsky PE, Hubbard RC, Talwalker S, Schwartz BD, et al. Preliminary study of the safety and efficacy of SC-58635, a novel cyclooxygenase 2 inhibitor: efficacy and safety in two placebo-controlled trials in osteoarthritis and rheumatoid arthritis, and studies of gastrointestinal and platelet effects. Arthritis Rheum. 1998; 41:1591–1602. PMID: 9751091.

8. Ehrich EW, Dallob A, De Lepeleire I, Van Hecken A, Riendeau D, Yuan W, et al. Characterization of rofecoxib as a cyclooxygenase-2 isoform inhibitor and demonstration of analgesia in the dental pain model. Clin Pharmacol Ther. 1999; 65:336–347. PMID: 10096266.

9. Singer E, Dionne R. A controlled evaluation of ibuprofen and diazepam for chronic orofacial muscle pain. J Orofac Pain. 1997; 11:139–146. PMID: 10332320.
10. Gottesdiener K, Mehlisch DR, Huntington M, Yuan WY, Brown P, Gertz B, et al. Efficacy and tolerability of the specific cyclooxygenase-2 inhibitor DFP compared with naproxen sodium in patients with postoperative dental pain. Clin Ther. 1999; 21:1301–1312. PMID: 10485502.

11. Ta LE, Dionne RA. Treatment of painful temporomandibular joints with a cyclooxygenase-2 inhibitor: a randomized placebocontrolled comparison of celecoxib to naproxen. Pain. 2004; 111:13–21. PMID: 15327804.

12. Yap AU, Tan KB, Chua EK, Tan HH. Depression and somatization in patients with temporomandibular disorders. J Prosthet Dent. 2002; 88:479–484. PMID: 12473996.

13. Falisi G, Rastelli C, Panti F, Maglione H, Quezada Arcega R. Psychotropic drugs and bruxism. Expert Opin Drug Saf. 2014; 13:1319–1326. PMID: 25195948.

14. Chang JP, Wu CC, Su KP. A case of venlafaxine-induced bruxism alleviated by duloxetine substitution. Prog Neuropsychopharmacol Biol Psychiatry. 2011; 35:307. PMID: 21111016.

15. Herman CR, Schiffman EL, Look JO, Rindal DB. The effectiveness of adding pharmacologic treatment with clonazepam or cyclobenzaprine to patient education and self-care for the treatment of jaw pain upon awakening: a randomized clinical trial. J Orofac Pain. 2002; 16:64–70. PMID: 11889661.
16. Rizzatti-Barbosa CM, Nogueira MT, de Andrade ED, Ambrosano GM, de Barbosa JR. Clinical evaluation of amitriptyline for the control of chronic pain caused by temporomandibular joint disorders. Cranio. 2003; 21:221–225. PMID: 12889679.

17. Hersh EV, Balasubramaniam R, Pinto A. Pharmacologic management of temporomandibular disorders. Oral Maxillofac Surg Clin North Am. 2008; 20:197–210. PMID: 18343325.

18. Ownby SL, Fortuno LV, Au AY, Grzanna MW, Rashmir-Raven AM, Frondoza CG. Expression of pro-inflammatory mediators is inhibited by an avocado/soybean unsaponifiables and epigallocatechin gallate combination. J Inflamm (Lond). 2014; 11:8. PMID: 24678847.

19. Christiansen BA, Bhatti S, Goudarzi R, Emami S. Management of osteoarthritis with avocado/soybean unsaponifiables. Cartilage. 2015; 6:30–44. PMID: 25621100.

20. Halon A, Donizy P, Dziegala M, Dobrakowski R, Simon K. Tissue laser biostimulation promotes post-extraction neoangiogenesis in HIV-infected patients. Lasers Med Sci. 2015; 30:701–706. PMID: 23917415.

21. Ahrari F, Madani AS, Ghafouri ZS, Tunér J. The efficacy of lowlevel laser therapy for the treatment of myogenous temporomandibular joint disorder. Lasers Med Sci. 2014; 29:551–557. PMID: 23318917.

22. Bradley P. The maxillofacial region: recent research and clinical practice in low intensity laser therapy. In : Simunovic Z, editor. Lasers in medicine and dentistry: basic science and up-to-date clinical application of low energy-level laser therapy--LLLT. Rijeka: European Medical Laser Association;2000. p. 385–402.
23. Kulekcioglu S, Sivrioglu K, Ozcan O, Parlak M. Effectiveness of low-level laser therapy in temporomandibular disorder. Scand J Rheumatol. 2003; 32:114–118. PMID: 12737331.

24. Coombe AR, Ho CT, Darendeliler MA, Hunter N, Philips JR, Chapple CC, et al. The effects of low level laser irradiation on osteoblastic cells. Clin Orthod Res. 2001; 4:3–14. PMID: 11553080.

25. Pinheiro AL, Cavalcanti ET, Pinheiro TI, Alves MJ, Miranda ER, De Quevedo AS, et al. Low-level laser therapy is an important tool to treat disorders of the maxillofacial region. J Clin Laser Med Surg. 1998; 16:223–226. PMID: 9796491.

26. Mannheimer JS. Therapeutic modalities. In : Kraus SL, editor. TMJ disorders management of the craniomandibular complex. New York: Churchill Livingstone;1988. p. 311–337.
27. Jankelson B, Radke JC. The myo-monitor: its use and abuse (I). Quintessence Int Dent Dig. 1978; 9:47–52.
28. Pereira LJ, Gavião MB, Bonjardim LR, Castelo PM, Andrade Ada S. Ultrasonography and electromyography of masticatory muscles in a group of adolescents with signs and symptoms of TMD. J Clin Pediatr Dent. 2006; 30:314–319. PMID: 16937858.

29. Ariji Y, Katsumata A, Hiraiwa Y, Izumi M, Sakuma S, Shimizu M, et al. Masseter muscle sonographic features as indices for evaluating efficacy of massage treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010; 110:517–526. PMID: 20868996.

30. El Fatih IA, Ibrahim AI, El Laithi A. Efficacy of physiotherapy and intraoral splint in the management of temporomandibular disorders. J Saudi Dent. 2004; 16:16–20.
31. Esposito CJ, Veal SJ, Farman AG. Alleviation of myofascial pain with ultrasonic therapy. J Prosthet Dent. 1984; 51:106–108. PMID: 6583380.

32. Santuzzi CH, Gonçalves WLS, Rocha SS, Castro MEC, Gouvea SA, Abreu GR. Effects of cryotherapy, transcutaneous electrical stimulation and their combination on femoral nerve electrical activity in rats. Rev Bras Fisioter. 2008; 12:441–446.
33. Deshpande RG, Mhatre S. TMJ disorders and occlusal splint therapy: a review. Int J Dent Clin. 2010; 2:22–29.
34. Gray RJ, Davies SJ. Occlusal splints and temporomandibular disorders: why, when, how? Dent Update. 2001; 28:194–199. PMID: 11476035.

35. Winocur E, Gavish A, Emodi-Perlman A, Halachmi M, Eli I. Hypnorelaxation as treatment for myofascial pain disorder: a comparative study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002; 93:429–434. PMID: 12029281.

36. Al-Ani MZ, Davies SJ, Gray RJ, Sloan P, Glenny AM. Stabilisation splint therapy for temporomandibular pain dysfunction syndrome. Cochrane Database Syst Rev. 2004; (1):CD002778. PMID: 14973990.

37. Qvintus V, Suominen AL, Huttunen J, Raustia A, Ylöstalo P, Sipilä K. Efficacy of stabilisation splint treatment on facial pain: 1-year follow-up. J Oral Rehabil. 2015; 42:439–446. PMID: 25644634.
38. Triantaffilidou K, Venetis G, Bika O. Efficacy of hyaluronic acid injections in patients with osteoarthritis of the temporomandibular joint. A comparative study. J Craniofac Surg. 2013; 24:2006–2009. PMID: 24220392.

39. Alpaslan GH, Alpaslan C. Efficacy of temporomandibular joint arthrocentesis with and without injection of sodium hyaluronate in treatment of internal derangements. J Oral Maxillofac Surg. 2001; 59:613–618. PMID: 11381380.

40. Long X, Chen G, Cheng AH, Cheng Y, Deng M, Cai H, et al. A randomized controlled trial of superior and inferior temporomandibular joint space injection with hyaluronic acid in treatment of anterior disc displacement without reduction. J Oral Maxillofac Surg. 2009; 67:357–361. PMID: 19138610.

41. Alpaslan C, Bilgihan A, Alpaslan GH, Güner B, Ozgür Yis M, Erbaş D. Effect of arthrocentesis and sodium hyaluronate injection on nitrite, nitrate, and thiobarbituric acid-reactive substance levels in the synovial fluid. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000; 89:686–690. PMID: 10846121.

42. Sato S, Kawamura H. Changes in condylar mobility and radiographic alterations after treatment in patients with non-reducing disc displacement of the temporomandibular joint. Dentomaxillofac Radiol. 2006; 35:289–294. PMID: 16798928.
43. Sato S, Nasu F, Motegi K. Analysis of kinesiograph recordings and masticatory efficiency after treatment of non-reducing disk displacement of the temporomandibular joint. J Oral Rehabil. 2003; 30:708–713. PMID: 12791156.

44. Sato S, Nasu F, Motegi K. Analysis of post-treatment electromyographs in patients with non-reducing disc displacement of the temporomandibular joint. J Oral Rehabil. 2002; 29:1126–1130. PMID: 12453269.

45. Basterzi Y, Sari A, Demirkan F, Unal S, Arslan E. Intraarticular hyaluronic acid injection for the treatment of reducing and nonreducing disc displacement of the temporomandibular joint. Ann Plast Surg. 2009; 62:265–267. PMID: 19240522.

46. Yeung RWK, Chow RLK, Samman N, Chiu K. Short-term therapeutic outcome of intra-articular high molecular weight hyaluronic acid injection for nonreducing disc displacement of the temporomandibular joint. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006; 102:453–461. PMID: 16997111.

47. Meunier FA, Schiavo G, Molgó J. Botulinum neurotoxins: from paralysis to recovery of functional neuromuscular transmission. J Physiol Paris. 2002; 96:105–113. PMID: 11755789.

48. The Wealthy Dentist [Internet]. Tiburon (CA): The Wealthy Dentist;cited 2018 Feb 6. Available from: http://thewealthydentist.com/.
49. Emara AS, Faramawey MI, Hassaan MA, Hakam MM. Botulinum toxin injection for management of temporomandibular joint clicking. Int J Oral Maxillofac Surg. 2013; 42:759–764. PMID: 23538215.

50. Arinci A, Güven E, Yazar M, Başaran K, Keklik B. Effect of injection of botulinum toxin on lateral pterygoid muscle used together with the arthroscopy in patients with anterior disk displacement of the temporomandibular joint. Kulak Burun Bogaz Ihtis Derg. 2009; 19:122–129. PMID: 19857189.
51. Kim HS, Yun PY, Kim YK. A clinical evaluation of botulinum toxin-A injections in the temporomandibular disorder treatment. Maxillofac Plast Reconstr Surg. 2016; 38:5. PMID: 26855937.

52. Moon YM, Kim YJ, Kim MK, Kim SG, Kweon H, Kim TW. Early effect of Botox-A injection into the masseter muscle of rats: functional and histological evaluation. Maxillofac Plast Reconstr Surg. 2015; 37:46. PMID: 26753166.

53. Sidebottom AJ, Patel AA, Amin J. Botulinum injection for the management of myofascial pain in the masticatory muscles. A prospective outcome study. Br J Oral Maxillofac Surg. 2013; 51:199–205. PMID: 22871559.

54. Park SY, Park YW, Ji YJ, Park SW, Kim SG. Effects of a botulinum toxin type A injection on the masseter muscle: an animal model study. Maxillofac Plast Reconstr Surg. 2015; 37:10. PMID: 25938091.

55. Song PC, Schwartz J, Blitzer A. The emerging role of botulinum toxin in the treatment of temporomandibular disorders. Oral Dis. 2007; 13:253–260. PMID: 17448205.

56. Monje-Gil F, Nitzan D, González-Garcia R. Temporomandibular joint arthrocentesis. Review of the literature. Med Oral Patol Oral Cir Bucal. 2012; 17:e575–e581. PMID: 22322493.

57. Cömert Kiliç S, Güngörmüş M, Sümbüllü MA. Is arthrocentesis plus platelet-rich plasma superior to arthrocentesis alone in the treatment of temporomandibular joint osteoarthritis? A randomized clinical trial. J Oral Maxillofac Surg. 2015; 73:1473–1483. PMID: 25976690.

58. Davis CL, Kaminishi RM, Marshall MW. Arthroscopic surgery for treatment of closed lock. J Oral Maxillofac Surg. 1991; 49:704–707. PMID: 2056368.

59. Stringer DE, Park CM. Single-cannula technique for operative arthroscopy using holmium:YAG laser. J Oral Maxillofac Surg. 2012; 70:49–50. PMID: 21982691.

60. McCain JP, Hossameldin RH. Advanced arthroscopy of the temporomandibular joint. Atlas Oral Maxillofac Surg Clin North Am. 2011; 19:145–167. PMID: 21878249.

61. Moon SY, Chung H. Ultra-thin rigid diagnostic and therapeutic arthroscopy during arthrocentesis: development and preliminary clinical findings. Maxillofac Plast Reconstr Surg. 2015; 37:17. PMID: 26191518.

62. Indresano AT. Surgical arthroscopy as the preferred treatment for internal derangements of the temporomandibular joint. J Oral Maxillofac Surg. 2001; 59:308–312. PMID: 11243614.

63. Machoň V, Sedý J, Klíma K, Hirjak D, Foltán R. Arthroscopic lysis and lavage in patients with temporomandibular anterior disc displacement without reduction. Int J Oral Maxillofac Surg. 2012; 41:109–113. PMID: 21885248.

64. Smolka W, Iizuka T. Arthroscopic lysis and lavage in different stages of internal derangement of the temporomandibular joint: correlation of preoperative staging to arthroscopic findings and treatment outcome. J Oral Maxillofac Surg. 2005; 63:471–478. PMID: 15789318.

65. Smolka W, Yanai C, Smolka K, Iizuka T. Efficiency of arthroscopic lysis and lavage for internal derangement of the temporomandibular joint correlated with Wilkes classification. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008; 106:317–323. PMID: 18226569.

66. McCain JP, Sanders B, Koslin MG, Quinn JH, Peters PB, Indresano AT. Temporomandibular joint arthroscopy: a 6-year multicenter retrospective study of 4,831 joints. J Oral Maxillofac Surg. 1992; 50:926–930. PMID: 1506966.

67. Murakami KI, Tsuboi Y, Bessho K, Yokoe Y, Nishida M, Iizuka T. Outcome of arthroscopic surgery to the temporomandibular joint correlates with stage of internal derangement: five-year follow-up study. Br J Oral Maxillofac Surg. 1998; 36:30–34. PMID: 9578253.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download