I. Introduction
Malignant melanomas are classified as mucosal and cutaneous melanomas
1. Mucosal malignant melanoma is a relatively rare disease compared to cutaneous melanoma that affects the head and neck region
2. Mucosal malignant melanoma accounts for 0.4% to 2% of all malignant melanomas and 10% of all head and neck melanomas
3. Involved sites of mucosal melanoma include the oral cavity, nasal cavity, paranasal sinus, nasopharynx, and larynx
45. Sinonasal mucosal melanoma (SNMM) accounts for 3.5% of all malignancy neoplasms found in the sinonasal region and accounts for 6.7% of head and neck melanomas
6. Melanoma arising from mucosa tends to be more aggressive and has a poorer prognosis than cutaneous melanoma
7. The 5-year survival rates of SNMM are reported to vary from 0% to 30%
8.
The main site of SNMM is the nasal cavity including the nasal septum and lateral nasal wall
9. Cases involving paranasal sinuses such as the maxillary sinus and ethmoid sinus are less common
10. Symptoms of SNMM include nasal blockages, facial pressure, and nasal blood discharge
11. The etiology of SNMM is unclear
10. Oral mucosa melanoma has a flat and thin appearance while SNMM manifests as an exophytic, bulky, and polypoid mass
4. More than one third of SNMMs are characterized by weak or no pigmentation
48. Consequently, diagnosis is difficult and often confused with other pathologies such as nasal polyposis and sinusitis
9. For an accurate diagnosis, excisional biopsies and histopathological examinations should be required
4. Immunohistochemical stains with S-100 and homatropine methylbromide-45 (HBM-45) have been performed to confirm diagnosis of SNMM in the head and neck region
12. We report a case of SNMM primarily occurring in a right maxillary sinus mucosa simulated as a cystic lesion. This case report was approved by the Institutional Review Board (IRB) of Gangneung-Wonju National University Dental Hospital, Gangneung, Korea (IRB 2017-002).
Go to :

II. Case Report
A 78-year-old female patient was referred from a local clinic for a cystic lesion on her right maxillary sinus. She had facial swelling on the right paranasal and maxillary molar area that formed 2 months prior. Swelling and fluctuation were observed in the right maxilla buccal mucosa without pigmentation.(
Fig. 1. A) She suffered from pain and a fever and had no specific medical history except for hypertension. Haziness in the right maxillary sinus was observed on panoramic view.(
Fig. 1. B) The large soft tissue mass was also observed via cone-beam computed tomography.(
Fig. 1. C) Cortical thinning and expansion of the sinus wall was observed. The medial wall of the sinus had deviated to the nasal cavity. Although the perforation of the cortical bone was observed on the anterior maxillary sinus wall, there was no destructive bony formation or invasive extension to the surrounding structure.(
Fig. 1. D).
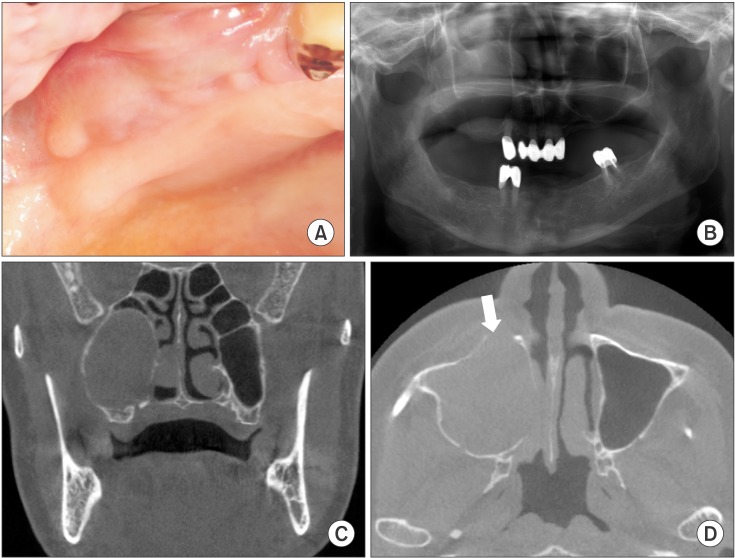 | Fig. 1Radiological and clinical images of the right maxillary sinus. A. Buccal mucosal swelling without pigmentation as result of tumor expansion. B. Haziness of the right maxillary sinus and an indistinct alveolar crestal line on panoramic view. C. Cortical bone thinning and expansion on the right maxillary sinus. D. Cortical bone perforation on the anterior wall of the maxillary sinus (white arrow).
|
The soft tissue mass was excised under general anesthesia. The mass extended from the floor of the maxillary sinus to the ostium of the maxillary sinus and medially to the nasal cavity. The tumor mass was well encapsulated within the epithelium and did not infiltrate the medial wall of the maxillary sinus.(
Fig. 2. A) The mass was easily separated from the expanded bony wall and was excised in 1 piece. The excised mass was greyish and brownish in color and weakly pigmented. The bony defect was covered with 2 layers of flaps that included the buccal mucosa and pedicled buccal fat pad (BFP).(
Fig. 2. B) The specimen included a greyish and brownish soft tissue mass 5.5×4.0 cm in size that was weakly pigmented.(
Fig. 2. A) The tissue mass contained an organized blood clot and hemorrhage.(
Fig. 2. A) On histopathological examination, neoplastic tumor cells with prominent nucleoli and malignant spindle cells were observed. Some tumor cells contained melanin.(
Fig. 3. A) Based on strongly positive immunohistochemistry results using S-100 and HBM-45, malignant mucosal melanoma was diagnosed.(
Fig. 3. B, 3. C) One month after the operation, the surgical site was completely healed without complications and we referred the patient for chemotherapy and radiotherapy treatment. However, she refused all additional treatments and only received some antibiotics.
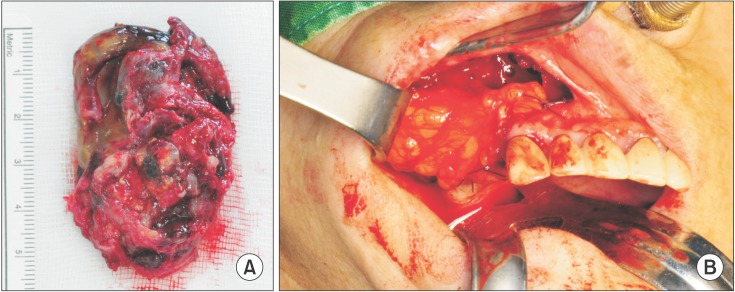 | Fig. 2A. Well encapsulated and partly pigmented soft tissue mass. B. Buccal fat pad graft after tumor excision.
|
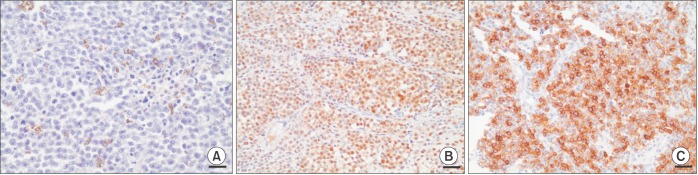 | Fig. 3Histological and immunohistochemical image. Melanin pigmentation and mitosis is observed (A; H&E staining, ×400, scale bar=25 µm). Immunohistochemistry with S-100 (B; ×200, scale bar=50 µm) and homatropine methylbromide-45 (C; ×200, scale bar=50 µm).
|
Go to :

IV. Discussion
SNMMs are malignant neoplasms found in the head and neck region
6. They typically form in the nasal septum and lateral nasal wall and later in the inferior turbinate, maxillary sinus, and ethmoid sinus
110. SNMMs developing from the paranasal sinus are rarely found
8. SNMMs have an ulcerative and necrotized surface of variable coloring depending on the characteristics of melanin pigmentation
10. They cause unspecific symptoms such as nasal obstructions, recurrent nasal epistaxis, and sometimes facial pressure
11. Recently, such symptoms have been detected in the maxillary sinus
11. Due to the nonspecific and late onset of these symptoms, diagnosis of maxillary sinus SNMM is difficult
1. In our case, the patient did not experience nasal epistaxis or obstruction. She exhibited facial swelling and fluctuations in the right maxillary buccal mucosa due to the mass.
SNMMs in the maxillary sinus grow aggressively and invasively
11. They extend invasively and infiltrate surrounding structures such as the nasal cavity, ethmoid sinus, and middle turbinate
1. Radiologically, SNMMs have an ill-defined border and show signs of bone destruction around a lesion
11. However, low-grade SNMMs can manifest as a polypoid mass without infiltrating lesions and are often confused with nasal polyps, chronic sinusitis, and inflammatory pseudotumors
9131415. In our case, a large soft tissue mass was observed on the right maxillary sinus with round appearance. Cortical bone thinning and mass expansion to the nasal cavity were observed.(
Fig. 1. C) Although a cortical bone perforation was observed on the anterior sinus wall, signs of bone destruction or irregular margins around the mass were not present.(
Fig. 1. D) Consequently, this case was difficult to identify and initially diagnosed as a cystic or benign lesion.
SNMMs are difficult to diagnose due to the presence of variable neoplastic cells
2. SNMMs have small blue round cells, pleomorphic and epithelioid cells, and spindle cells
1016. Due to the presence of round blue cells, SNMMs can be confused with lymphomas, olfactory neuroblastomas, or small cell carcinomas
10. Pleomorphic and epithelioid cells may be misdiagnosed as carcinoma. Melanoma spindle cells are also often confused as sarcomas or spindle cell carcinomas
9. Malignant melanoma is diagnosed based on the presence of melanin pigmentation in tumor cells
1. However, more than one third of SNMMs lack pigmentation and are referred to as amelanotic melanomas
14. For these reasons, immunohistochemical staining is necessary for the diagnosis of SNMM.
Expressions of S-100 and HBM-45 were used as diagnostic markers for melanoma
17. S-100 protein is derived from Schwann cells, melanocytes, and myoepithelial cells. The protein is found in cases of melanoma, schwannoma, and neurofibroma
16. HMB-45 is a monoclonal antibody that reacts with the antigen derived from melanoma
16. Histologically, neoplastic tumor cells were mostly dispersed and grew with prominent nucleoli. Furthermore, melanin pigmentation was observed in the cytoplasm of tumor cells.(
Fig. 3. A) Based on immunohistochemistry analysis, the specimens were positive for S-100 and HBM45.(
Fig. 3. B, 3. C) Consequently, the patient was diagnosed with primary SNMM from the maxillary sinus mucosa.
SNMMs have a poorer survival rate when found in the maxillary and ethmoid sinuses than in the nasal cavity
11. SNMMs in the maxillary sinus are closer to the skull base and can easily infiltrate the lamina papyracea
11. A malignancy of the skull base and orbit is associated with poor prognosis
18. Fortunately, our case did not involve the skull base but instead expansion to the ostium and anterior wall of the maxillary sinus.(
Fig. 1. C) Standard SNMM treatment involves broad surgical excision with negative margins
10. Depending on the size of the tumor and the degree of lymphatic invasion additional chemotherapy and radiation therapy may be required
119. In our case, the tumor was well encapsulated and thus was easily excised; the defect was covered with pedicle BFP to prevent fistula formation.(
Fig. 2. B) BFP grafting is a useful method for covering intraoral defects after tumor resection
20.
In our case, a primary SNMM was found arising from the maxillary sinus mucosa simulating a cystic lesion. We diagnosed the SNMM based on the histological features of melanin pigmentation in the neoplastic tumor cells. The final diagnosis was established via immunohistochemistry with S-100 and HMB-45.
Go to :






 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download