Abstract
Orbital hypertelorism is an increased distance between the bony orbits and can be caused by frontonasal malformations, craniofacial clefts, frontoethmoidal encephaloceles, glial tumors or dermoid cysts of the root of the nose, and various syndromic or chromosomal disorders. We report a series of 7 cases of hypertelorism that were treated in our hospital. The underlying causes in our series were craniofacial clefts 0 to 14 (4 cases), craniofacial clefts 1 to 12 (1 case), and frontonasal encephalocele (2 cases), all congenital. Surgical techniques used to correct the deformity were box osteotomy and medial wall osteotomy with or without calvarial and rib grafts. A few of our cases were reoperations with specific challenges.
Embryologically, the development of orbital hypertelorism has been linked to deficient remodeling of the nasal capsule or premature closure of the sphenofrontal suture12. Craniofacial surgery to correct hypertelorism has seen many modifications and improvements during the past few decades. Habal3 summarized the journey, which he grouped into three generations, G1, G2, and G3. In G1, routine sacrifice of the olfactory nerve and resection of the cribriform plate were performed. G2 saw preservation of the cribriform plate, and in G3, orbital surgery minimizes the involvement of the maxilla to prevent maxillary complications. The rapid advancements have been aided by the development of cutting drills for osteotomies.
Orbital hypertelorism is a disorder of the facial skeleton in which there is increased distance between the bony orbits45. More specifically, the distance is increased between the two dacrya, the junction of the lacrimal bone, the frontal process of the maxilla, and the frontal bone6. The normal distance is approximately 28 mm in an adult female and 32 mm in an adult male7. Tessier8 classified orbital hypertelorism into 3 categories; grade I (30–34 mm), grade II (35–39 mm), and grade III (>40 mm). A grade III deformity involves gross facial disfigurement and requires surgical intervention. The grade of severity indicates the need for surgical correction, and the etiology and type of hypertelorism influence the selection of operative procedure. Orbital hypertelorism is a congenital deformity subsequent to a malformation.
The timing of the surgery should take into consideration the anatomical and functional aspects, along with the more important psychological benefits for a growing child with an increased awareness of and exposure to the outside world. The corrective surgeries depend on the degree of deficiency and the underlying cause. A set of 5 diagnostic criteria has been proposed for the diagnosis of hypertelorism: frontonasal malformations, craniofrontonasal dysplasias, craniofacial clefts, encephaloceles, and a miscellaneous group that includes syndromic or chromosomal disorders9. The origin of the deformity guides the selection of surgical treatment. Orbital medialization depends on the axis of the orbits. If the bony orbits are widely displaced, then the corrective procedure will involve a box osteotomy; however, in cases of increased intercanthal distance, a medial osteotomy is sufficient. If there are occlusal alterations, then the treatment will be facial bipartition with rotation of the hemifaces. The most common surgical approach is intracranial, though a subcranial approach can be performed in cases with less severe deformity.
Ethical approval was taken from Combined Military Hospital Kharian Cantt's ethical committee (no. 12/2017). The written informed consent was obtained from all patients.
We present a series of 7 cases, 5 female and 2 male patients, between the ages of 4 and 26 years. Of the 7 cases, 2 cases involved reoperation. The preoperative work up included an assessment of previous records and consultations, where available. Investigations included skull posteroanterior and lateral radiographs and a frontal cephalogram and its interpretation by an orthodontist. The orthodontist advised the surgical team regarding the required corrections when formulating the treatment objectives. Clinical measurements were conducted using a Vernier caliper/scale, and intraoral and extraoral photographs were taken of all the patients as part of preoperative workup. A computed tomography (CT) scan with three-dimensional (3D) reconstruction was performed to assess the bony deformity and for future planning. Magnetic resonance (MR) imaging was also done to observe any encephaloceles or other unsuspected features of the malformation. An ophthalmological examination, including fundoscopy, was done for all the patients. A neurosurgical consultation was always done, and the patients were discussed in a joint meeting of a craniofacial surgeon, neurosurgeon, oral and maxillofacial surgeon, anesthetist, and orthodontist. The ophthalmologist was called only if there was an ophthalmological finding, which in our series was not required. The preanesthesia assessment was performed by the anesthetist after completion of the preoperative workup. One patient had corpus callosum lipoma on the preoperative MR scan. The case series consists of 1 case of nasal reconstruction with box osteotomy, 1 case of medial wall osteotomy with a calvarial bone graft for nasal and medial orbital wall reconstruction, 1 case of forehead remodeling with a box osteotomy, and 4 cases of box osteotomy as a single procedure.
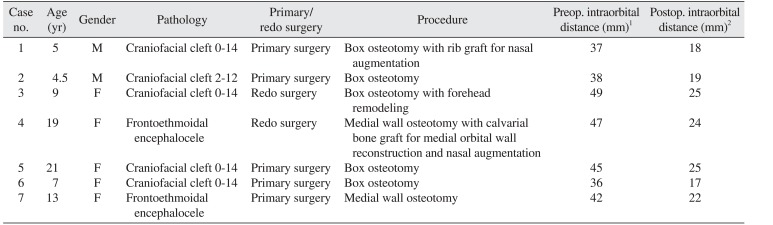
We followed the same protocol for all the patients. An orotracheal cuffed tube was placed. Arterial access was obtained for invasive monitoring. Six units of red cell concentrate were arranged for all patients, irrespective of age, and were available on demand. Coronal access was gained through a zigzag coronal incision. Osteotomies were performed using an electric powered drill system. The operation time ranged from 9 to 16 hours. A 1.5-mm microplate system was used in all the cases, along with the use of fibrin glue and bone dust. A pericranial flap was raised in fresh cases. No flap was available in the redo cases, so sub-periosteal access was made using coronal and subciliary incisions. A frontal craniotomy was done with preservation of a 1 cm frontal bar; in 1 case, it was only partially available. The frontal bar served as a landmark for orbital osteotomies and orbital box osteotomies. The osteotomies were carried out between 15 and 17 mm behind the orbital margin. Osteotomy from the orbital roof and that from medial and lateral walls were carried out with an oscillating saw, whereas orbital floor osteotomy was conducted with a combination of an oscillating saw and an osteotome. The craniofacial surgeon harvested calvarial bone grafts for bone gaps. Canthopexy was performed in all patients using a 0.35-mm stainless steel wire. Two non-suction drains were placed over the coronal aspect and were removed once the drainage fell below 15 mL per 24 hours. The patients were kept in intensive care, propped up to 30° for the first 2 days, after which they were moved to the surgical ward. There was no mortality or major infection, though 2 patients had a prolonged cerebro-spinal fluid (CSF) leak. One of those patients was lost to follow-up, and the other recovered uneventfully on conservative measures. The antibiotic regime consisted of amikacin (15 mg/kg/day) and vancomycin (10 mg/kg every 6 hours daily intravenous) for 7 days, with generous use of narcotic analgesics. No major dural tears were encountered during surgery. No neurological deficit was detected. The 3 patients who showed preoperative anosmia required secondary operations. The follow-up protocol consisted of a visit at 3 weeks, then 3 months, and then yearly afterward. The follow-up ranged from 2 to 6 years. Table 1 describes the patient details, the deformity for which the surgery was performed, the technique used, and the preoperative and postoperative measurements.
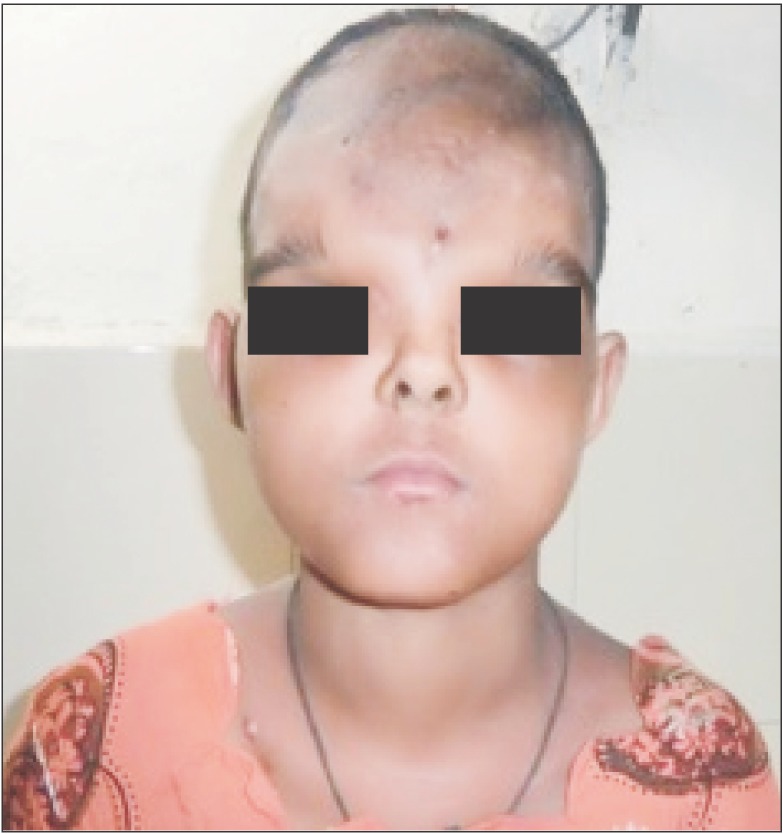
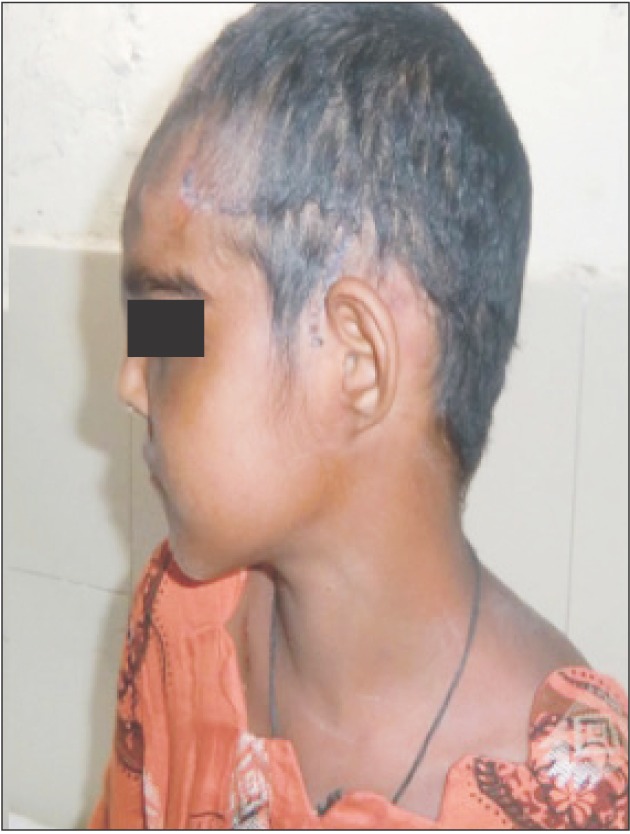
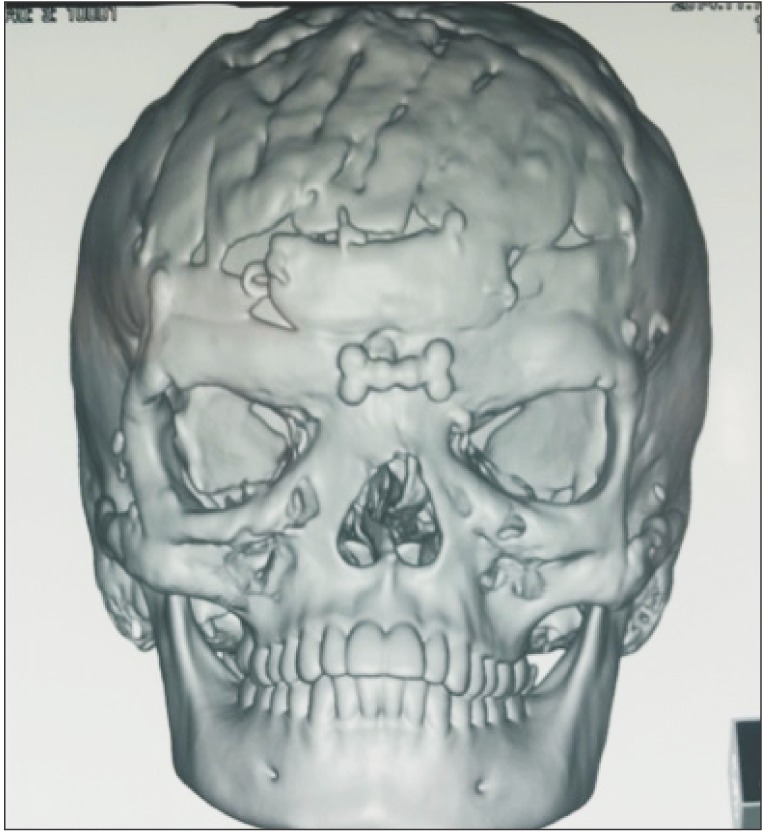
Fig. 1 and 2 show the preoperative picture/CT scan of a female patient aged 9 years. She has a visible forehead scar from a neurosurgical procedure carried out previously. Fig. 3 is a preoperative picture of the correction for hypertelorism and forehead remodeling with autologous cranial bone. Fixation was conducted with a combination of wire and osteosynthesis plates. Fig. 4 and 5 show the postoperative frontal and lateral pictures of the same patient. Fig. 6 is the postoperative 3D CT scan showing the bone graft in place for the frontal defect.
Orbital hypertelorism is an etiologically heterogeneous disorder found in various craniofacial anomalies10. Tessier8 classified orbital hypertelorism in adults into grades I, II, and III using interorbital distance measurements. Surgical correction should be considered in patients with grade II/III. In children, hypertelorism can be graded using standard deviations from age- and gender-matched data11. It has been suggested that orbital hypertelorism develops due to arrest of the first branchial arch, which ordinarily leads to herniation of the brain forward and downward between the orbits to prevent growth. The etiology of craniofacial clefts can be intrinsic or extrinsic. Extrinsic causes include amniotic bands, and intrinsic causes are radiation, influenza, toxoplasmosis, metabolic abnormalities, and certain drugs, such as anticonvulsants12. According to Tessier et al.10 and Converse et al.13, the enlargement of ethmoid cells and bone is the cause of orbital hypertelorism. Other surgeons suggest that hypoplasia and premature closure of the suture lines at the base of the skull and in the region anterior to the sphenoid bone are the main cause4. We now clearly know that orbital hypertelorism is always associated with congenital craniofacial anomalies.
The surgical techniques we used were box osteotomy and medial orbital osteotomy, depending on the deformity and ultimate treatment goal. In addition, we performed forehead remodeling and nasal augmentation using a rib graft or split calvarial graft. We did not use the U-shaped osteotomy technique because it is suitable only for grade I and grade II orbital hypertelorism. One of the claimed benefits of that technique is the shortening of the operation time by 2 hours and a marked reduction in perioperative bleeding1415.
Of the many variations in surgical techniques, one is the medial hemiorbital advancement or a medial canthopexy, in addition to medial translocation of the medial orbital walls161718. Another option is medial translocation of the medial orbital walls with medial canthopexy as a single procedure19202122. In frontoethmoidal encephalocele, facial bipartition could be a more appropriate choice of procedure. A narrow arch with inverted incisors and orbits that are downward/oblique also require facial bipartition surgery. The ideal age at which to undergo surgery for orbital hypertelorism remains under debate, but it can be effectively managed as early as 6 to 8 years of age.
McCarthy et al.23 summed up the age-related issues about operating for hypertelorism. According to him, the best results are achieved in patients who have finished growing. In those patients, the orbital bones are thicker and allow stable fixation. Thin bones complicate medial canthoplexy. Some of the complications that surgeons have reported include infection, persistent CSF leakage, hematoma, bleeding, optic nerve damage, relapse of bone/soft tissue deformity, bone graft resorption, bone graft extrusion, extrusion of wires, hypersensitivity reactions from drugs and blood products, need for tracheostomy, pneumothorax, and death. The complications we encountered were two cases of postoperative CSF leak that responded to conservative measures. One patient had a head injury due to a fall, with resulting resorption of the forehead craniotomy panel.
Our series had no mortality, and the longest follow-up was 6 years. All patients were assessed postoperatively for their outcomes, and all patients responded positively about improvements in facial profile. The need for redo surgeries complicates everything about treating this condition; the planning, access, osteotomies, perioperative bleeding, and postoperative complications, such as CSF leak.
Many centers around the world use 3D printed models for surgeries to correct hypertelorism. One such case was reported by Engel et al.24, in which they carried out surgery on a 3D printed model using cuts corresponding to the proposed surgical osteotomies. In addition to 3D printing, stereolithographic models are used by some surgeons. It is generally said that 3D printing is superior to stereolithographic models25 because the 3D printed models can be planned in terms of osteotomy, bone grafts, and tactile sense before even touching the patient. Another modality for treatment is virtual planning, in which computer software is used to virtually manipulate images in line with the planned surgery to predict the final outcome26. Due to a lack of 3D printing facilities and stereolithographic models, we had to rely on 3D CT scanning and MR images.
In conclusion, orbital hypertelorism is a difficult deformity to correct, especially in its severe form. Every patient harbors a unique deformity requiring astute planning and execution. There is no textbook approach to the problem, and a multidisciplinary team adds to the good results that can be achieved. Every patient needs a special plan tailored to his/her needs and realistically achievable objectives, keeping in view the old surgical dictum to “do no harm.”
Acknowledgements
We acknowledge all the operation room staff without whom these surgeries weren't possible. We also acknowledge the efforts of all the people who have helped in the treatment planning, literature review, manuscript writing and last but not the least the Anesthesiologists and the Neurosurgeons without whom the surgeries weren't possible.
References
1. Vermeij-Keers C, Mazzola RF, Van der Meulen JC, Strickler M. Cerebro-craniofacial and craniofacial malformations: an embryological analysis. Cleft Palate J. 1983; 20:128–145. PMID: 6406099.
2. Vermeij-Keers C, Poelmann RE, Smits-Van Prooije AE, Van der Meulen JC. Hypertelorism and the median cleft face syndrome. An embryological analysis. Ophthalmic Paediatr Genet. 1984; 4:97–105. PMID: 6545389.

3. Habal MB. Surgical correction of orbital hypertelorism: a surgical evolution through time. J Craniofac Surg. 2009; 20:715–717. PMID: 19461324.
4. Edgerton MT, Udvarhelyi GB, Knox DL. The surgical correction of ocular hypertelorism. Ann Surg. 1970; 172:473–496. PMID: 4918004.

5. Lloyd LA. Craniofacial reconstruction: ocular management of orbital hypertelorism. Trans Am Ophthalmol Soc. 1975; 73:123–140. PMID: 1246801.
6. AO Foundation. Diagnosis of hypertelorism [Internet]. Davos: AO Foundation;cited 2016 Oct 19. Available from: https://www2.aofoundation.org.
7. Tessier P. Anatomical classification facial, cranio-facial and laterofacial clefts. J Maxillofac Surg. 1976; 4:69–92. PMID: 820824.
8. Tessier P. Orbital hypertelorism I Successive surgical attempts Material and methods Causes and mechanisms. Scand J Plast Reconstr Surg. 1972; 6:135–155. PMID: 4652235.

9. Tan ST, Mulliken JB. Hypertelorism: nosologic analysis of 90 patients. Plast Reconstr Surg. 1997; 99:317–327. PMID: 9030136.

10. Tessier P, Guiot G, Rougerie J, Delbet JP, Pastoriza J. Cranionaso-orbito-facial osteotomies. Hypertelorism. Ann Chir Plast. 1967; 12:103–118. PMID: 5595910.
11. Liu DL, Shan L, Yuan Q, Huang JJ. Refinement of the correction of orbital hypertelorism. J Craniofac Surg. 2011; 22:217–219. PMID: 21233750.

12. Morovic CG, Berwart F, Varas J. Craniofacial anomalies of the amniotic band syndrome in serial clinical cases. Plast Reconstr Surg. 2004; 113:1556–1562. PMID: 15114114.

13. Converse JM, Ransohoff J, Mathews ES, Smith B, Molenaar A. Ocular hypertelorism and pseudohypertelorism. Advances in surgical treatment. Plast Reconstr Surg. 1970; 45:1–13. PMID: 5409842.

14. Mühling J, Zöller J, Saffar M, Reinhart E, Reuther J. Results of operative therapy of bony orbit dystopies. Mund Kiefer Gesichtschir. 1998; 2(Suppl 1):S94–S97.
15. Shen W, Cui J, Chen J, Ying ZX. Treatment of orbital hypertelorism using inverted U-shaped osteotomy. J Craniofac Surg. 2015; 26:415–417. PMID: 25699535.

16. Mahapatra AK, Tandon PN, Dhawan IK, Khazanchi RK. Anterior encephaloceles: a report of 30 cases. Childs Nerv Syst. 1994; 10:501–504. PMID: 7882371.

18. Mahapatra AK, Suri A. Anterior encephaloceles: a study of 92 cases. Pediatr Neurosurg. 2002; 36:113–118. PMID: 11919444.

19. Mahatumarat C, Rojvachiranonda N, Taecholarn C. Frontoethmoidal encephalomeningocele: surgical correction by the Chula technique. Plast Reconstr Surg. 2003; 111:556–565. PMID: 12560676.

20. Mahatumarat C, Taecholarn C, Charoonsmith T. One-stage extracranial repair and reconstruction for frontoethmoidal encephalomeningocele: a new simple technique. J Craniofac Surg. 1991; 2:127–133. discussion 134. PMID: 1814492.
21. Rojvachiranonda N, Mahatumarat C, Taecholarn C. Correction of the frontoethmoidal encephalomeningocele with minimal facial incision: modified Chula technique. J Craniofac Surg. 2006; 17:353–357. PMID: 16633187.
22. Charoonsmith T, Suwanwela C. Frontoethmoidal encephalomeningocele with special reference to plastic reconstruction. Clin Plast Surg. 1974; 1:27–47. PMID: 4426155.

23. McCarthy JG, La Trenta GS, Breitbart AS, Zide BM, Cutting CB. Hypertelorism correction in the young child. Plast Reconstr Surg. 1990; 86:214–225. PMID: 2367571.

24. Engel M, Hoffmann J, Castrillon-Oberndorfer G, Freudlsperger C. The value of three-dimensional printing modelling for surgical correction of orbital hypertelorism. Oral Maxillofac Surg. 2015; 19:91–95. PMID: 25249178.

25. Cohen A, Laviv A, Berman P, Nashef R, Abu-Tair J. Mandibular reconstruction using stereolithographic 3-dimensional printing modeling technology. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009; 108:661–666. PMID: 19716728.

26. Xie K, Yang S, Zhu YM. 3D visualization and simulation in surgical planning system of orbital hypertelorism. J Med Syst. 2011; 35:617–623. PMID: 20703527.

Fig. 1
Clinical photograph of a 9-year-old girl of grade III hypertelorism with a history of previous neurosurgical procedure (craniotomy incision can be seen.).
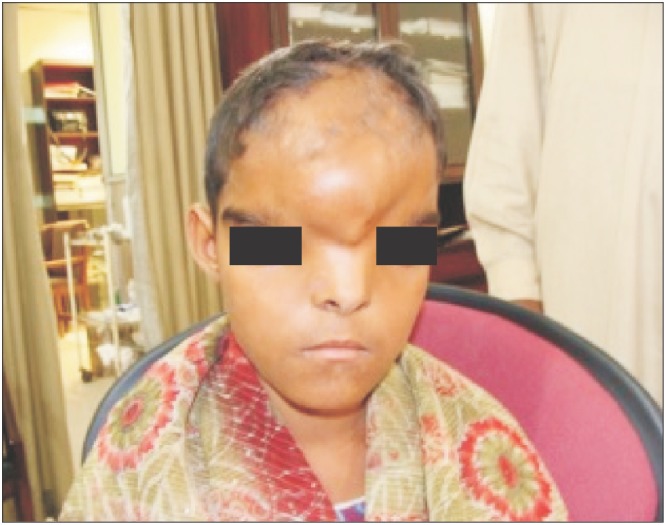
Fig. 2
Preoperative three-dimensional reconstruction of the same patient showing frontal bone defect and grade III hypertelorism.
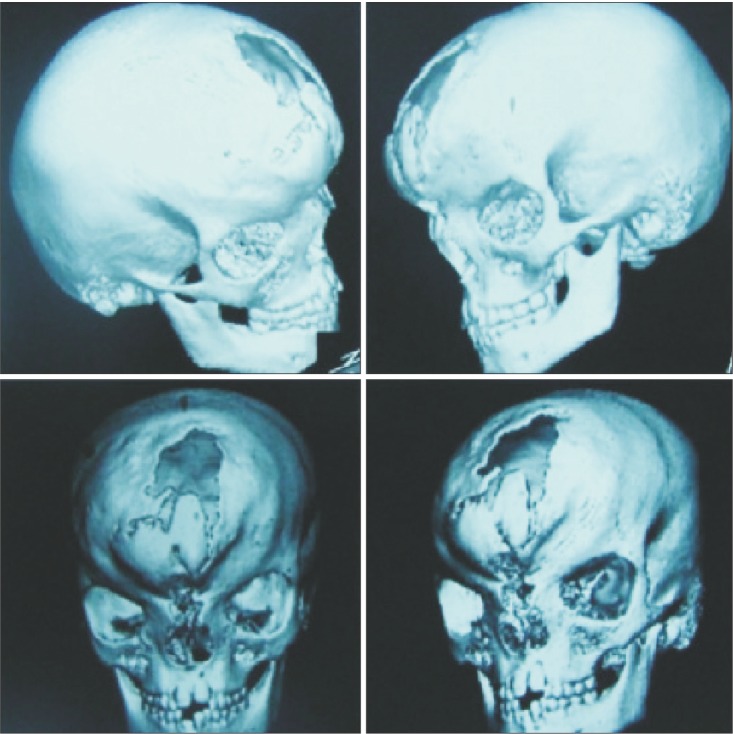
Fig. 3
Peroperative picture of correction of hypertelorism and forehead remodeling with autologous cranial bone. Fixation was done with stainless steel wire and osteosynthesis.
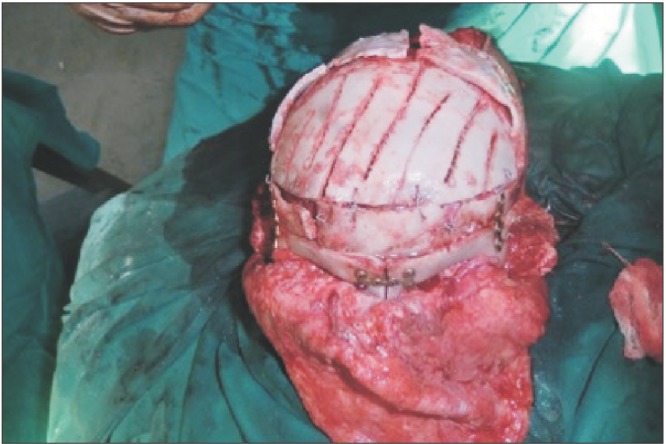
Table 1
The patient details, the deformity for which the surgery was performed, the technique used, and the preop. and postop. interorbital distance




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download