This article has been
cited by other articles in ScienceCentral.
Abstract
Objectives
Headache is the most common complaint of patients suffering from temporomandibular joint disorders (TMDs). Thus, temporomandibular joint (TMJ) examinations maybe necessary in patients with headache. Considering the high prevalence of bruxism and TMDs in patients with headache the effects of conservative TMD treatment on headache should be assessed.
Materials and Methods
Patients were questioned about headaches in the past three months. Those responding affirmatively to this question were examined for TMD and bruxism. After the examinations, 219 patients remained in the study and received self-management instructions. Patients were requested to modify oral habits except when eating or sleeping. The degree of pain (visual analogue scale), headache disability index (HDI), frequency of headaches (FH) per month and TMD intensity were evaluated.
Results
The median levels of pain, HDI, FH, and TMD intensity were 8, 44, 8, and 7, respectively, before modifying oral habits and decreased to 4, 24, 2, and 3, respectively, after intervention. These decreases were statistically significant.
Conclusion
Having patients maintain free space between the teeth and relax muscles can be an efficient method to treat headache and TMD, especially when repeated frequently.
Go to :

Keywords: Headache, Temporomandibular joint disorders, Para-functional habits, Self-care
I. Introduction
Headache is a costly disorder with a high prevalence, affecting over 80% of the population. It inflicts a high load on health care systems. Headache is the most common complaint in patients with temporomandibular joint disorders (TMDs) and, in some cases, may be the sole symptom
1. Pain due to TMD is the most common oral pain for which patients often seek treatment from a dentist
2.
TMDs are almost always multi-factorial. Para-functional activities are an important cause of TMDs since they overload the masticatory system and teeth. Among the para-functional activities in the stomatognathic system, bruxism is a major risk factor for the initiation and continuation of TMD due to both muscular and articular conditions
3. Despite the high prevalence of bruxism among patients with headache, researchers found that bruxism alone does not increase the risk of primary headache; however, when associated with TMDs, it increases the risk of different types of headaches. Based on this finding, a hypothesis was proposed that links TMDs, bruxism and primary headache
4.
Considering these associations, headache treatment requires an interdisciplinary approach
5. Dentists can play an important role by treating temporomandibular joint (TMJ) problems
6. Among the available TMD treatments, behavioral interventions, such as reversing habits with self-management instructions, are conservative, affordable and efficient approaches. Patients should be aware that para-functional habits do not change spontaneously, and the patients themselves are responsible for changing these behaviors
7. This study aimed to assess the effect of treating bruxism with self-care instructions and patient education on the severity and FH in TMD patients.
Go to :

II. Materials and Methods
This was an interventional phase II clinical trial. This trial was conducted in the Department of Oral and Maxillofacial Medicine, School of Dentistry, Tehran University of Medical Sciences (Tehran, Iran) in 2015 and was registered on Iranian Registry of Clinical Trials (IRCT; IRCT201410091559N7). The study protocol was approved by the ethics committee of the School of Dentistry, Tehran University of Medical Sciences. Patients presenting to the Department of Oral and Maxillofacial Medicine for the first time for oral and dental examinations were randomly questioned about headaches in the past three months. Those responding affirmatively to this question (336 patients) were briefed about the study design, its duration and the required cooperation and were enrolled in the study if they consented to participate by signing a written informed consent form.
Inclusion criteria for the study were age from 18 to 65 years, suffering from headaches for the past three months or longer, primary headache including migraine or tension headache in the opinion of a neurologist, headache severity of three or more on a visual analogue scale (VAS), having TMD (muscular or articular) and para-functional habits (grinding or clenching). A total of 219 patients were enrolled, of which 199 patients (28 males and 171 females) with a mean age of 36.5±9.9 years remained until the end of follow-up.
Helkimo's clinical dysfunction index was used to determine TMD and assess the severity of TMJ functional problems on the basis of five clinical criteria: mandibular mobility, muscle pain, TMJ pain, and pain during mandibular movement
8.
To assess para-functional habits, both sleeping and waking habits must be evaluated. According to clinical diagnostic indices recommended by the American Academy of Sleep Science, sleep bruxism is diagnosis when the patient is aware of it and the patient or someone sleeping next to the patient reports the sound of teeth grinding during sleep. This confirmation along with at least one of the following signs can confirm sleep bruxism: abnormal tooth wear (especially on the incisal edges or cusp tips); morning pain, stiffness or fatigue in the muscles of mastication; hypertrophy of the masseter muscle on palpation and activity of the muscles of mastication not explained by other sleep disorders, neurological or medical disorders, medication use or illicit drug use. For waking bruxism, definite diagnostic criteria have not yet been introduced, and a diagnosis is made based on observations and tooth sounds
9.
To assess clenching, patients were asked to close their mouth at rest. After a few moments, the lips were retracted and teeth contact noted. Also, the presence of scalloping on the lateral margins of the tongue (crenation), which indicates excessive tongue pressure on the teeth, was evaluated.
Three different methods were used to assess headache. (1) A set of questions based on the 2nd edition of the International Headache Classification (ICHD-II) was designed to determine the type of headache. By using this questionnaire and patient examination, a neurologist determined the headache type and frequency. (2) A VAS was used to assess pain. The patients were asked to determine the severity of headache on a 0- to 10-point scale where 0 indicated no pain and 10 indicated maximum pain. Headache severity was also assessed with a descriptive scale: mild, moderate, and severe. (3) The headache disability index (HDI) questionnaire was used. This questionnaire was designed by Jacobson et al.
10 in 1994.
Self-management instructions: Patients were asked to place their tongue behind the maxillary anterior teeth as when saying “N” and then close their mouth leaving a few millimeters of space between the occluding surfaces of the maxillary and mandibular teeth when the mandible was in a resting position (free space) with the lips in slight contact and relaxed. Patients were instructed to maintain this relaxed position at rest at all times except when eating or speaking and avoid unwanted muscle contraction with the teeth in centric occlusion Patients were scheduled for a follow-up after two months. During this period, several methods were used to remind patients of these instructions. A text message was sent to patients every other day. Alarms and reminders were set on the patients' cell phones. Two phone calls were also made to check on patients and make sure they were complying with the instructions and encourage them to continue. At the follow-up session, headache and TMD assessments were repeated, and the results were compared with baseline findings. Those changing their medications during this period, starting a new treatment (except for our instructions), not receiving the text messages, not adhering to the self-management instructions for any reason, those with incorrect phone numbers, not returning for follow-up, or developing a condition affecting the severity of headache were excluded.
Statistical analysis was done with SPSS software ver. 16 (SPSS Inc., Chicago, IL, USA). Data were analyzed with Wilcoxon, Mann-Whitney and Kruskal-Wallis tests. P<0.05 was considered significant.
Go to :

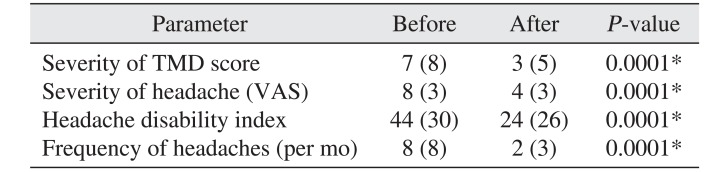
III. Results
Wilcoxon test showed that the median TMD severity score, headache severity, HDI, and FH decreased significantly after modifying habits (
P=0.0001).(
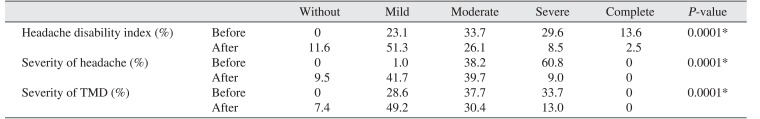
Table 1) Also, modifying bruxism affected descriptive TMD and headache scales.(
Table 2)
Table 1
Comparison of variables before and after oral para-functional habit modification
|
Parameter |
Before |
After |
P-value |
|
Severity of TMD score |
7 (8) |
3 (5) |
0.0001*
|
|
Severity of headache (VAS) |
8 (3) |
4 (3) |
0.0001*
|
|
Headache disability index |
44 (30) |
24 (26) |
0.0001*
|
|
Frequency of headaches (per mo) |
8 (8) |
2 (3) |
0.0001*
|

Table 2
Changes in descriptive TMD and headache scales following para-functional habit modification
|
Without |
Mild |
Moderate |
Severe |
Complete |
P-value |
|
Headache disability index (%) |
Before |
0 |
23.1 |
33.7 |
29.6 |
13.6 |
0.0001*
|
|
After |
11.6 |
51.3 |
26.1 |
8.5 |
2.5 |
|
Severity of headache (%) |
Before |
0 |
1.0 |
38.2 |
60.8 |
0 |
0.0001*
|
|
After |
9.5 |
41.7 |
39.7 |
9.0 |
0 |
|
Severity of TMD (%) |
Before |
0 |
28.6 |
37.7 |
33.7 |
0 |
0.0001*
|
|
After |
7.4 |
49.2 |
30.4 |
13.0 |
0 |

The change in bruxism did not differ significantly between males and females.(
Table 3)
Table 3
Changes in variables following para-functional habit modification between males and females

|
Female (n=171) |
Male (n=28) |
P-value |
|
Severity of TMD score |
2 (4) |
1 (3) |
0.403 |
|
Severity of headache (VAS) |
4 (2) |
4 (3) |
0.744 |
|
Headache disability index |
18 (18) |
19 (21) |
0.894 |
|
Frequency of headaches (per mo) |
5 (8) |
5 (8) |
0.982 |

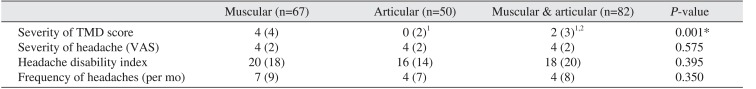
The median TMD severity differed significantly among muscular, articular and combined types of TMD as determined with a Kruskal-Wallis test (
P=0.001).(
Table 4) It was significantly higher in Muscular TMD was significantly more severe than both articular and combined TMD, and combined TMD was significantly more severe than articular TMD.
Table 4
Changes in variables following para-functional habit modification according to TMD type
|
Muscular (n=67) |
Articular (n=50) |
Muscular & articular (n=82) |
P-value |
|
Severity of TMD score |
4 (4) |
0 (2)1
|
2 (3)1,2
|
0.001*
|
|
Severity of headache (VAS) |
4 (2) |
4 (2) |
4 (2) |
0.575 |
|
Headache disability index |
20 (18) |
16 (14) |
18 (20) |
0.395 |
|
Frequency of headaches (per mo) |
7 (9) |
4 (7) |
4 (8) |
0.350 |

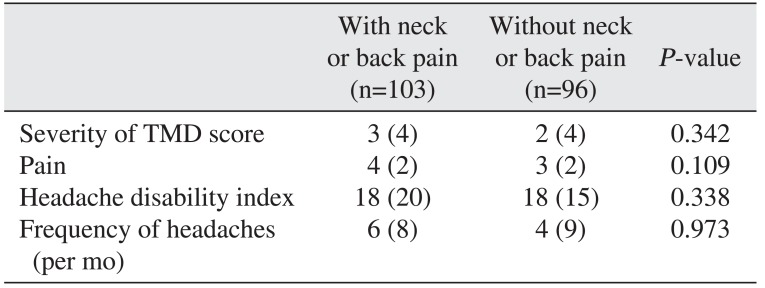
The change in bruxism did not differ significantly between patients with or without neck or back pain.(
Table 5)
Table 5
Changes in variables following para-functional habit modification in patients with or without neck or back pain
|
With neck or back pain (n=103) |
Without neck or back pain (n=96) |
P-value |
|
Severity of TMD score |
3 (4) |
2 (4) |
0.342 |
|
Pain |
4 (2) |
3 (2) |
0.109 |
|
Headache disability index |
18 (20) |
18 (15) |
0.338 |
|
Frequency of headaches (per mo) |
6 (8) |
4 (9) |
0.973 |

Go to :

IV. Discussion
Human beings adapt to prolonged stressful conditions (para-typical factors) through a complex collection of metabolic factors, spirit and memory, including sensitivity, integrity and motivation. These behavioral factors act together to effect growth and development. To affect their environment, humans must control their muscle contractions. The stomatognathic system includes proprioception of the muscles of mastication and the tongue, along with lingual, occlusal, articular, mucosal, cutaneous and autosomal nerves. Sensory afferents of deep occlusal nerves balance occlusion between the right and left dental quadrants. They also balance the deglutition and bilateral mastication. However, under some painful or stressful conditions, occlusal afferents cannot adequately transfer signals and lead to abnormal motor reflexes (occlusal problems, impaired tongue function). This mechanism may result in atypical deglutition and temporary or permanent unilateral mastication. Such atypical movements may lead to continuous spasm of the stomatognathic system. Thus, under stressful conditions, some adaptive disorders may develop. In general, if stressful conditions are not well controlled, severe and continuous muscle spasms during the day and night may follow and result in para-functional habits damaging the stomatognathic system.
Isotonic contractions related to phasic function of the muscles of mastication results in grinding and isometric contraction related to tonic muscle function maintains clenching. Several studies have reported that bruxism can be the most destructive para-functional activity for the stomatognathic system and is considered the main risk factor for TMD
311. The effect of bruxism on muscles can be explained as follows. Pain in 0 the muscles of mastication occurs due to impaired blood supply. Swelling occurs due to increased intramuscular pressure and continuous activation of several motor units with a low threshold for excessive load. Damage to these muscles results in inflammation, pain and tenderness. Delayed onset muscle soreness due to ultra-structural destruction of muscle fibers and connective tissue that lead to delayed inflammatory responses has also been observed in animal studies
4. The effect of bruxism on the joint can be explained as follows. Continuous muscle force due to clenching compresses the disc, which leads to creep deformity of the disc and eventually generates shear stress. Shear stress increases nitric oxide release, which is involved in the pathogenesis of joint disorders as a reactive oxygen metabolite. Increased nitric oxide is related to increased chondrocyte apoptosis
9. Epidemiologic studies have shown that TMD causes chronic pain, which can be related to other chronic pain such as headache, neck pain, shoulder pain and backache. Headache is the most common TMD symptom (22%); while 55% of patients with chronic headache complain of TMD signs and symptoms
12.
TMD has been shown to correlate with headache
13. Para-functional habits, mediated by TMDs, can increase the risk, frequency and severity of primary headaches
4. The main hypothesis proposes that the pain is due to the central sensitization phenomenon, in which a neuron transferring a pain signal to the central nervous system stimulates adjacent neurons. This phenomenon is caused by (1) long-term neurotransmitter accumulation at the synapses of continuous afferent inputs, which leads to neurotransmitter leakage and excitation of adjacent interstitial and (2) the convergence-projection hypothesis in which many afferent neurons synapse with an interstitial neuron. Under normal conditions, the cortex differentiates the site of pain, but presence of continuous, deep pain confuses the cortex and results in the sensation of pain in normal structures. Convergence-projection synapses of other deep tissues are present in the sensory nucleus of the trigeminal nerve, known as silent connections, and are turned on only when receiving nociceptive inputs from muscles due to a decreased repetitive compressive pain threshold. Primary sensitization of nociceptive afferent neurons in muscles is known as peripheral sensitization
14. Thus, both peripheral and central sensitization are required for headaches to originate from the muscles of mastication.
Another hypothesis to explain muscle pain is the muscle spindle hypothesis. To maintain balance, axial muscles of the body (head, neck, and trunk) contract simultaneously, and the contraction of each muscle is associated with that of other muscles. Thus, in patients who maintain facial muscle contraction, muscle spindle receptors are stimulated and motor centers in the face, neck and the trunk are stimulated, resulting in muscle contraction in these areas. Decreased blood supply to these muscles results in spasm and pain in the long-term
15. TMD can initiate, aggravate or prolong all types of headaches
14. Thus, occlusal appliances should be prescribed as a reminder and to decrease teeth contacts and muscle tension. Self-care along with education is among the most fundamental, conservative and cost-effective strategies to treat TMDs
16. This method is efficient since TMD is a self-limiting condition. The human brain can learn new behaviors, and frequent exercise and repetition further establish these behaviors. Thus, this treatment can be suitable for both sleeping and waking bruxism. In this study, after two months of treatment, the TMD severity, headache intensity, disability due to headache (qualitative and quantitative) and headache frequency decreased significantly. This indicated a strong correlation between para-functional habits and headache development and aggravation.
Most previous studies assessed the effect of self-management only on TMD intensity and not headaches. For instance, Kalamir et al.
17 assessed the efficacy of self-care compared to manual and splint therapy and reported similar efficacies. Michelotti et al.
18 assessed the combination of self-care with other methods such as manual and exercise therapy and reported that combined therapies were more effective than self-care alone. None of the studies on simultaneous TMD treatment and headache reported the use of self-care. Ekberg and Nilner
19 studied the efficacy of intraoral appliances for treating headache and TMD. In 2013, effect of manual therapy on the severity of headache and TMD was evaluated
20. To our knowledge, the current study is the first to assess the efficacy of self-care for simultaneously treating TMD and headache. This method, in contrast to pharmaceutical therapy, does not have any side effects, drug interference, or risk of overdose. It is free and patients do not need to present to medical centers or undergo costly imaging or therapeutic procedures. The efficacy of this method is good, and the results are stable as long as the patient continues the self-management practices. Some of the methodological limitations in our study were the large number of patients participating in this study, confirmation of inclusion criteria for all patients, time consuming examinations and questionnaires, frequent reminders provided to patients and patient recall for follow-up examination.
Go to :

V. Conclusion
Having patients maintain free space between the teeth and relax muscles can be an efficient method to treat headache and TMD, especially when repeated frequently.
Go to :










 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download