Abstract
Objectives
TNM staging, especially for lymph node metastasis, is the scoring system most widely used among prognostic factors for cancer survival. Several biomarkers have been studied as serologic markers, but their specificity is low and clinical applications are difficult. This study aimed to establish a scoring system for patients with oral squamous cell carcinoma (OSCC) using platelet (PLT) and mean platelet volume (MPV) levels measured postoperatively and to evaluate their significance as prognostic factors.
Materials and Methods
We studied 40 patients admitted to the Department of Oral and Maxillofacial Surgery of Dankook University Hospital who were diagnosed with primary OSCC histopathologically between May 2006 and May 2012. Clinical pathological information obtained from the medical records of each patient included age, sex, height, weight, tumor location, degree of differentiation, tumor diameter, lymph node metastasis, TNM stage, and other test values including white blood cell, MPV, PLT, C-reactive protein (CRP), and albumin obtained through a test conducted within 7 days before surgery. Count of platelet (COP)-MPV Score: Patients with both PLT and MPV values below the cut-off values were defined as score 0 (group A). Patients with at least one of the two higher than the cut-off value were defined as score 1 (group B).
Results
Univariate analyses showed N-metastasis, COP-MPV (A vs B), PLT, platelet-lymphocyte ratio, and CRP were statistically significant prognostic factors. A multivariate Cox proportional hazards model showed N-metastasis (hazard ratio [HR] 6.227, P=0.016) and COP-MPV (A vs B) (HR 18.992, P=0.013) were independent prognostic factors with a significant effect on survival.
The number of cases of carcinoma of the lips and oral cavity worldwide was about 300,000 in 2012, or 2.1% of all carcinomas that year12. Among them, squamous cell carcinoma was most common among males aged over 60 years who drank and smoked345. In recent years, however, the incidence has been increasing in women 40 years and below who do not have these same cancer risk factors678.
Oral squamous cell carcinoma (OSCC) is treated with surgery, radiotherapy, and chemotherapy, often in combination. For patients in early TNM stages I and II, the effect of a single surgical treatment or radiotherapy was not statistically significant9. However, for patients in a more advanced TNM stage (III or IV), the combination of surgical and radiotherapy showed the best therapeutic effect10.
The TNM staging, especially for lymph node metastasis, is the scoring system most widely used among prognostic factors for patient survival1112. Several biomarkers have been studied as serologic markers, but their specificity is low and clinical applications are difficult13. It is essential that clinicians discover effective and clinically efficient biomarkers as prognostic factors for OSCC.
In studies conducted over the last few decades, platelet activation has been considered an important biological process for cancer occurrence and metastasis141516. Platelet (PLT) and mean platelet volume (MPV) are the most common measures of platelet activation17, and high MPV levels indicate abnormal platelet production and activation18. Recent studies have shown that MPV levels are relatively high in tumor patients, and that prognosis was poor in patients with gastric tumors showing relatively high MPV levels192021. There have been no studies conducted on the application of both PLT and MPV level in predicting postoperative survival in patients with OSCC.
This study aimed to establish a scoring system for OSCC patients using PLT and MPV levels measured postoperatively and to evaluate their significance as prognostic factors.
We studied 40 patients admitted to the Department of Oral and Maxillofacial Surgery of Dankook University Hospital (Cheonan, Korea) who were diagnosed with primary OSCC histopathologically between May 2006 and May 2012. Patient cases were excluded for any of the following reasons: (1) experienced severe complications or died within 30 days after surgery, (2) experienced preoperative systemic inflammatory response syndrome, (3) had infections or autoimmune diseases, or (4) had recently undergone radiotherapy or chemotherapy. A total of 40 patient cases were included in the study.
Clinical pathological information obtained from the medical records of each patient included age, sex, height, weight, tumor location, degree of differentiation, tumor diameter, lymph node metastasis, and TNM stage. TNM stage was diagnosed according to the American Joint Committee on Cancer (AJCC) 7th edition and other test values including white blood cell (WBC), MPV, PLT, C-reactive protein (CRP), and albumin obtained through a test conducted within 7 days before surgery. We conducted this study in compliance with the principles of the Declaration of Helsinki. Due to the retrospective nature of this study, it was granted written exemption by the Dankook University Dental Hospital's Institutional Review Board. The informed consent was waived.
The cut-off values of PLT and MPV were determined by receiver operating characteristic (ROC) curves. Groups were divided as described below. The cut-off value for PLT was 263.5×109 L−1 and for MPV was 10.15 fL. Patients with both PLT and MPV values below cut-off were defined as score 0 (group A). Patients with at least one of the two higher than cut-off were defined as score 1 (group B).
Neutrophil-lymphocyte ratio (NLR) and platelet-lymphocyte ratio (PLR) were calculated by dividing absolute neutrophil and PLT by absolute lymphocyte count.
Overall survival was defined as the period of time to the patient's death or the period of time to the date when last follow-up was performed. In order to compare significance between groups, categorical variables were expressed as a percentage using the χ2 test and continuous variables that were normally distributed were expressed through regularity tests. For continuous variables that did not follow a standard distribution, the mean of the two groups was compared using the Mann-Whitney U test and then expressed as mean±standard deviation. Cut-off values for all continuous variables were determined using ROC curves. Variables with P-values less than 0.05 were considered significant prognostic factors through univariate analysis. The effect of these variables on survival rate was analyzed using a multivariate Cox proportional hazards model. Kaplan-Meier curves and log-rank tests were used to determine the difference in survival rate between groups. IBM SPSS Statistics 21.0 software (IBM Co., Armonk, NY, USA) was used to conduct statistical analyses. P-values less than 0.05 were considered statistically significant.
A total of 40 patients (28 males and 12 females) were included in this study. The mean age at the time of diagnosis was 64.08±11.59 years (median age, 66 years; range, 35–88 years). The mean follow-up period was 32.58±22.49 months (range, 3.7–82.0 months) and 11 patients died during the observation period. For TNM stage, 10 patients (25.0%) were stage I, 7 patients (17.5%) were stage II, 6 patients (15.0%) were stage III, and 17 patients (42.5%) were stage IV. Primary tumors were located on the lips in 2 patients (5.0%), on the buccal mucosa in 3 patients (7.5%), on the floor of the mouth in 3 patients (7.5%), on the gum in 12 patients (30.0%), in the maxillary sinus in 2 patients (5.0%), and on the retromolar pad in 4 patients (10.0%). Mean PLT and MPV were 243.93±61.382 ×109 L−1 (103–389 ×109 L−1) and 10.05±0.67 fL (8.8–11.4 fL), respectively. Other parameter values and ranges were as follows: WBC, 6.44±1.79 ×103/µL (3.52–10.83 ×103/µL); albumin, 4.14±0.45 g/dL (2.6–4.8 g/dL); CRP, 0.52±0.79 mg/dL (0.02–4.61 mg/dL); PLR, 127±39.51 (37.73–252.46); NLR, 1.92±0.76 (0.53–3.89); and maximum tumor size, 2.75±1.36 cm (0.8–5.9 cm).
The mean age at the time of clinicopathological characteristic measurement for COP-MPV group A was older (68.13±6.67 years) than group B (61.64±13.20 years) (P=0.047). The COP-MPV group A was in the study longer on average (50.55±15.49 months) than the COP-MPV group B (36.64±20.13 months) (P=0.019). There was no statistically significant relationship with age (P=0.311), N-metastasis (P=1.000), or TNM stage (P=0.735).(Table 1)
The PLT of the COP-MPV group A was 216.33±25.87 and that of the COP-MPV group B was higher 260.48±70.50 (P=0.008). The MPV level of the COP-MPV group A (9.68±0.34) was lower than that of COP-MPV group B (10.27±0.72) (P=0.005). There was no statistically significant relationship with NLR (P=0.679), PLR (P=0.267), WBC (P=0.525), CRP (P=0.600), or albumin (P=0.738).(Table 2)
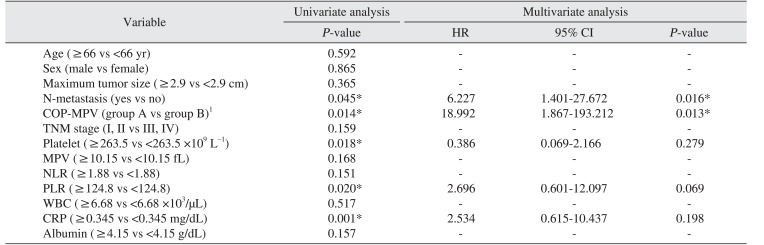
Clinical and laboratory variables were divided into groups based on their respective cut-off values and the relationship between overall survival and each respective variable was analyzed. Univariate analyses showed N-metastasis (P=0.045), COP-MPV (A vs B) (P=0.014), PLT (≥263.5 vs <263.5 ×109 L−1) (P=0.018), PLR (≥124.8 vs <124.8) (P=0.020), and CRP (≥0.345 vs <0.345 mg/dL) (P=0.001) were statistically significant prognostic factors.(Table 3)
A multivariate Cox proportional hazards model was used with univariate analyses to determine the significance of variables with a P-value less than 0.05 as independent prognostic factors for OSCC patients. Results were as follows: N-metastasis (HR 6.227, 95% confidence interval [CI] 1.401–27.672, P=0.016); COP-MPV (A vs B) (HR 18.992, 95% CI 1.867–193.212, P=0.013), indicating both N-metastasis and COP-MPV were significant independent prognostic factors for OSCC patient survival.(Table 3)
Kaplan-Meier analyses were used to determine the difference in overall survival between COP-MPV groups. The prognosis of COP-MPV group B was worse than that of COP-MPV group A (P=0.014). The prognosis of the group with lymph node metastasis was worse than that of the non-metastasis group (P=0.018).(Fig. 1, 2)
Recent studies have provided evidence that platelet activation is clinically significant in some malignant tumors. In particular, in a series of processes in which cancer cells spread to other organs through blood circulation, platelets are important for cancer cells to aggregate and be discharged from blood vessels2223. Some studies have reported that, in cancer patients, platelet activation indexes (soluble P-selectin, soluble CD40 ligand, and platelet factor 4) are relatively higher than in normal patients242526. Some studies have reported that MPV is also closely related to various thromboembolic disorders including ischemic cardiovascular disease2728, and some studies have reported that MPV is related to malignant tumors1929. However, whether the prognosis of patients with relatively high MPV is worse than those with low MPV remains a subject of debate. Osada et al.30 reported that MPV level rose in malicious tumors such as gastric cancer. In contrast, Mutlu et al.31 did not find any elevation in MPV level in various cancer patients and, compared with time of first cancer diagnosis, MPV level was significantly reduced at the time of thrombotic events. However, larger platelets secrete more chemical mediators upon stimulation than smaller ones, and the increase in number of larger platelets suggests that stimulation exists from diseases such as cardiovascular disease or malignant tumors272830. PLT and MPV are important indexes for assessing platelet activation state17. It is essential to predict the prognosis of OSCC patients using a scoring system that includes comprehensive consideration of PLT and MPV.
The mean age of group A was higher (68.13 years) than group B (61.64 years), indicating that age was lower in group B with poor prognosis (P=0.047).(Table 1) Goldstein and Irish32 and Sarkaria and Harari33 found in their study that squamous cell carcinoma in the head and neck showed a more aggressive tendency in younger age groups. In contrast, Veness et al.34 reported that it was difficult to predict prognosis by age35. However, in recent years, the incidence of OSCC is increasing in younger age groups363738.
In overall period, the COP-MPV group A was longer (50.55 months) than the COP-MPV group B (36.64 months) (P=0.019). There was no statistically significant relationship between COP-MPV score and sex, tumor location, N-metastasis, and TNM stage.(Table 1)
Multivariate analysis showed N-metastasis and COP-MPV were statistically significant independent prognostic factors. The N-metastasis group had a 6.227 times higher risk of death than the non-metastasis group (HR 6.227, 95% CI 1.401–27.672) and the risk of death in COP-MPV group B was 18.992 times higher than COP-MPV group A (HR 18.992, 95% CI 1.867–193.212, P=0.013). These results indicate that COP-MPV score had a greater effect on survival prognosis for OSCC patients than N-metastasis.
During the observation period, the survival rate of COP-MPV group B (56%) was significantly lower than that of COP-MPV group A (93.4%). The survival rate of the N-metastasis group was 50% and the survival rate of the non-metastasis group was 76.7%. Kaplan-Meier analysis showed the survival rate in the N-metastasis group was not better than that of the non-metastasis group (Fig. 2), and there was no statistically significant difference in survival rates between the group with maximum tumor size larger than the cut-off value and the group with maximum tumor size not larger than the cut-off value (P=0.365). Zhang et al.39 reported a 5-year survival rate of 20.5% in the N-metastasis group for OSCC patients and 72.3% in the non-metastasis group, claiming that N-metastasis was a more important prognostic factor for predicting survival rate than T stage. Through Cox regression, this study has shown that COP-MPV score (P=0.013) is a more meaningful prognostic factor than N-metastasis (P=0.016) for predicting the survival rate of OSCC patients. These results are similar to those of Zhang et al.40 regarding esophageal squamous cell carcinoma patients.
Khandavilli et al.41 reported that patients with OSCC with high preoperative CRP levels had poor prognosis, and that patients in TNF stage III and IV had relatively higher preoperative CRP levels than patients in stage I and II. Gockel et al.42 reported that lymph node metastasis and tumor extension increased in patients with higher CRP levels, and Park et al.43 reported that survival rate was low in patients with a high CRP/albumin ratio. Similar to these results, CRP (≥0.345 vs <0.345) (P=0.001) was a statistically significant prognostic factor on univariate analysis in this study. However, on multivariate analysis, CRP (P=0.198) was not an independent prognostic factor. These results indicate that the stratifying ability of CRP was likely incorporated in the COP-MPV score.
Kaplan-Meier analysis showed the survival rate of COP-MPV group A was higher than group B indicating that, if both COP and MPV were lower than the cut-off value, postoperative prognosis would be better.
Univariate analysis showed the P-value for PLT was 0.018 and the P-value for MPV was 0.168. PLT was a statistically significant prognostic factor alone but MPV alone was not. However, COP-MPV score (P=0.014) combining PLT and MPV was a more significant prognostic factor than PLT (P=0.018). These results suggest that COP-MPV score, which considers PLT and MPV in a comprehensive manner, is helpful for predicting the prognosis for OSCC patients.
This study has several limitations. First, the sample size is relatively small and it was a retrospective study conducted at a single institution. The initial hypothesis predicted that prognosis would be worse if both COP and MPV were higher than the cut-off value, but it was difficult to confirm this statistically because of the small sample size. Furthermore, the cut-off values for COP and MPV set in this study are not generally applicable to all patients with OSCC. In this study, COP-MPV score had more statistically significant results than other prognostic factors in determining OSCC patient prognosis. Future prospective studies with more subjects using COP-MPV score as a prognostic factor are needed to identify the applicable cut-off value for all patients with OSCC.
This study established that COP-MPV score was statistically significant for predicting the prognosis of 40 patients with OSCC. Multivariate analysis showed the survival rate of COP-MPV group B was lower than group A and COP-MPV score (HR 18.992, P=0.013) was found to be a more significant independent prognostic factor than N-metastasis (HR 6.227, P=0.016). COP-MPV score is a simple and cost-effective test method, and is considered a more effective factor relative to other considered factors in predicting OSCC patient prognosis.
References
1. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013; 63:11–30. PMID: 23335087.

2. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015; 136:E359–E386. PMID: 25220842.

3. Johnson N. Tobacco use and oral cancer: a global perspective. J Dent Educ. 2001; 65:328–339. PMID: 11336118.

4. Reibel J. Tobacco and oral diseases. Update on the evidence, with recommendations. Med Princ Pract. 2003; 12(Suppl 1):22–32. PMID: 12707498.
5. Lewin F, Norell SE, Johansson H, Gustavsson P, Wennerberg J, Biörklund A, et al. Smoking tobacco, oral snuff, and alcohol in the etiology of squamous cell carcinoma of the head and neck: a population-based case-referent study in Sweden. Cancer. 1998; 82:1367–1375. PMID: 9529030.
6. Llewellyn CD, Johnson NW, Warnakulasuriya KA. Risk factors for squamous cell carcinoma of the oral cavity in young people--a comprehensive literature review. Oral Oncol. 2001; 37:401–418. PMID: 11377229.
7. Singh B, Bhaya M, Zimbler M, Stern J, Roland JT, Rosenfeld RM, et al. Impact of comorbidity on outcome of young patients with head and neck squamous cell carcinoma. Head Neck. 1998; 20:1–7. PMID: 9464945.

8. Schantz SP, Yu GP. Head and neck cancer incidence trends in young Americans, 1973-1997, with a special analysis for tongue cancer. Arch Otolaryngol Head Neck Surg. 2002; 128:268–274. PMID: 11886342.

9. Lydiatt DD, Robbins KT, Byers RM, Wolf PF. Treatment of stage I and II oral tongue cancer. Head Neck. 1993; 15:308–312. PMID: 8360052.

10. Layland MK, Sessions DG, Lenox J. The influence of lymph node metastasis in the treatment of squamous cell carcinoma of the oral cavity, oropharynx, larynx, and hypopharynx: N0 versus N+. Laryngoscope. 2005; 115:629–639. PMID: 15805872.

11. Forastiere A, Koch W, Trotti A, Sidransky D. Head and neck cancer. N Engl J Med. 2001; 345:1890–1900. PMID: 11756581.

12. Woolgar JA, Rogers S, West CR, Errington RD, Brown JS, Vaughan ED. Survival and patterns of recurrence in 200 oral cancer patients treated by radical surgery and neck dissection. Oral Oncol. 1999; 35:257–265. PMID: 10621845.

13. Yotsukura S, Mamitsuka H. Evaluation of serum-based cancer biomarkers: a brief review from a clinical and computational viewpoint. Crit Rev Oncol Hematol. 2015; 93:103–115. PMID: 25459666.

14. Lip GY, Chin BS, Blann AD. Cancer and the prothrombotic state. Lancet Oncol. 2002; 3:27–34. PMID: 11908507.

15. Nash GF, Turner LF, Scully MF, Kakkar AK. Platelets and cancer. Lancet Oncol. 2002; 3:425–430. PMID: 12142172.

16. Gay LJ, Felding-Habermann B. Contribution of platelets to tumour metastasis. Nat Rev Cancer. 2011; 11:123–134. PMID: 21258396.

17. Kamath S, Blann AD, Lip GY. Platelet activation: assessment and quantification. Eur Heart J. 2001; 22:1561–1571. PMID: 11492985.

18. Kai H, Kitadai Y, Kodama M, Cho S, Kuroda T, Ito M, et al. Involvement of proinflammatory cytokines IL-1beta and IL-6 in progression of human gastric carcinoma. Anticancer Res. 2005; 25:709–713. PMID: 15868900.
19. Kurt M, Onal IK, Sayilir AY, Beyazit Y, Oztas E, Kekilli M, et al. The role of mean platelet volume in the diagnosis of hepatocellular carcinoma in patients with chronic liver disease. Hepatogastroenterology. 2012; 59:1580–1582. PMID: 22683976.
20. Karaman K, Bostanci EB, Aksoy E, Kurt M, Celep B, Ulas M, et al. The predictive value of mean platelet volume in differential diagnosis of non-functional pancreatic neuroendocrine tumors from pancreatic adenocarcinomas. Eur J Intern Med. 2011; 22:e95–e98. PMID: 22075321.

21. Kılınçalp S, Ekiz F, Başar O, Ayte MR, Coban S, Yılmaz B, et al. Mean platelet volume could be possible biomarker in early diagnosis and monitoring of gastric cancer. Platelets. 2014; 25:592–594. PMID: 23537073.

22. Connolly GC, Phipps RP, Francis CW. Platelets and cancer-associated thrombosis. Semin Oncol. 2014; 41:302–310. PMID: 25023346.

23. Stegner D, Dütting S, Nieswandt B. Mechanistic explanation for platelet contribution to cancer metastasis. Thromb Res. 2014; 133(Suppl 2):S149–S157. PMID: 24862136.

24. Caine GJ, Lip GY, Stonelake PS, Ryan P, Blann AD. Platelet activation, coagulation and angiogenesis in breast and prostate carcinoma. Thromb Haemost. 2004; 92:185–190. PMID: 15213860.

25. Li L, Li P, Yang YQ, Zhang H, Ai P, Wang F, et al. sCD40L, sP-selectin and sICAM-1 plasma levels in nasopharyngeal carcinoma. Sichuan Da Xue Xue Bao Yi Xue Ban. 2009; 40:513–516. PMID: 19627017.
26. Peterson JE, Zurakowski D, Italiano JE Jr, Michel LV, Connors S, Oenick M, et al. VEGF, PF4 and PDGF are elevated in platelets of colorectal cancer patients. Angiogenesis. 2012; 15:265–273. PMID: 22402885.

27. Azab B, Torbey E, Singh J, Akerman M, Khoueiry G, McGinn JT, et al. Mean platelet volume/platelet count ratio as a predictor of long-term mortality after non-ST-elevation myocardial infarction. Platelets. 2011; 22:557–566. PMID: 21714700.

28. Slavka G, Perkmann T, Haslacher H, Greisenegger S, Marsik C, Wagner OF, et al. Mean platelet volume may represent a predictive parameter for overall vascular mortality and ischemic heart disease. Arterioscler Thromb Vasc Biol. 2011; 31:1215–1218. PMID: 21330610.

29. Cho SY, Yang JJ, You E, Kim BH, Shim J, Lee HJ, et al. Mean platelet volume/platelet count ratio in hepatocellular carcinoma. Platelets. 2013; 24:375–377. PMID: 22835043.

30. Osada J, Rusak M, Kamocki Z, Dabrowska MI, Kedra B. Platelet activation in patients with advanced gastric cancer. Neoplasma. 2010; 57:145–150. PMID: 20099978.

31. Mutlu H, Artis TA, Erden A, Akca Z. Alteration in mean platelet volume and platicrit values in patients with cancer that developed thrombosis. Clin Appl Thromb Hemost. 2013; 19:331–333. PMID: 22345488.

32. Goldstein DP, Irish JC. Head and neck squamous cell carcinoma in the young patient. Curr Opin Otolaryngol Head Neck Surg. 2005; 13:207–211. PMID: 16012243.

33. Sarkaria JN, Harari PM. Oral tongue cancer in young adults less than 40 years of age: rationale for aggressive therapy. Head Neck. 1994; 16:107–111. PMID: 8021128.

34. Veness MJ, Morgan GJ, Sathiyaseelan Y, Gebski V. Anterior tongue cancer: age is not a predictor of outcome and should not alter treatment. ANZ J Surg. 2003; 73:899–904. PMID: 14616566.

35. Popovtzer A, Shpitzer T, Bahar G, Marshak G, Ulanovski D, Feinmesser R. Squamous cell carcinoma of the oral tongue in young patients. Laryngoscope. 2004; 114:915–917. PMID: 15126756.

36. Patel SC, Carpenter WR, Tyree S, Couch ME, Weissler M, Hackman T, et al. Increasing incidence of oral tongue squamous cell carcinoma in young white women, age 18 to 44 years. J Clin Oncol. 2011; 29:1488–1494. PMID: 21383286.

37. Marocchio LS, Lima J, Sperandio FF, Corrêa L, de Sousa SO. Oral squamous cell carcinoma: an analysis of 1,564 cases showing advances in early detection. J Oral Sci. 2010; 52:267–273. PMID: 20587952.

38. Chow CW, Tabrizi SN, Tiedemann K, Waters KD. Squamous cell carcinomas in children and young adults: a new wave of a very rare tumor? J Pediatr Surg. 2007; 42:2035–2039. PMID: 18082703.

39. Zhang WB, Wang Y, Mao C, Guo CB, Yu GY, Peng X. Cervical metastasis of maxillary squamous cell carcinoma. Int J Oral Maxillofac Surg. 2015; 44:285–291. PMID: 25467737.

40. Zhang F, Chen Z, Wang P, Hu X, Gao Y, He J. Combination of platelet count and mean platelet volume (COP-MPV) predicts postoperative prognosis in both resectable early and advanced stage esophageal squamous cell cancer patients. Tumour Biol. 2016; 37:9323–9331. PMID: 26779631.

41. Khandavilli SD, Ceallaigh PO, Lloyd CJ, Whitaker R. Serum C-reactive protein as a prognostic indicator in patients with oral squamous cell carcinoma. Oral Oncol. 2009; 45:912–914. PMID: 19502100.

42. Gockel I, Dirksen K, Messow CM, Junginger T. Significance of preoperative C-reactive protein as a parameter of the perioperative course and long-term prognosis in squamous cell carcinoma and adenocarcinoma of the oesophagus. World J Gastroenterol. 2006; 12:3746–3750. PMID: 16773693.

43. Park HC, Kim MY, Kim CH. C-reactive protein/albumin ratio as prognostic score in oral squamous cell carcinoma. J Korean Assoc Oral Maxillofac Surg. 2016; 42:243–250. PMID: 27847731.

Fig. 1
Kaplan-Meier curves of overall survival between count of platelet and mean platelet volume (COP-MPV) scores 0 and 1.

Table 1
Clinical and pathological characteristics between COP-MPV groups A and B
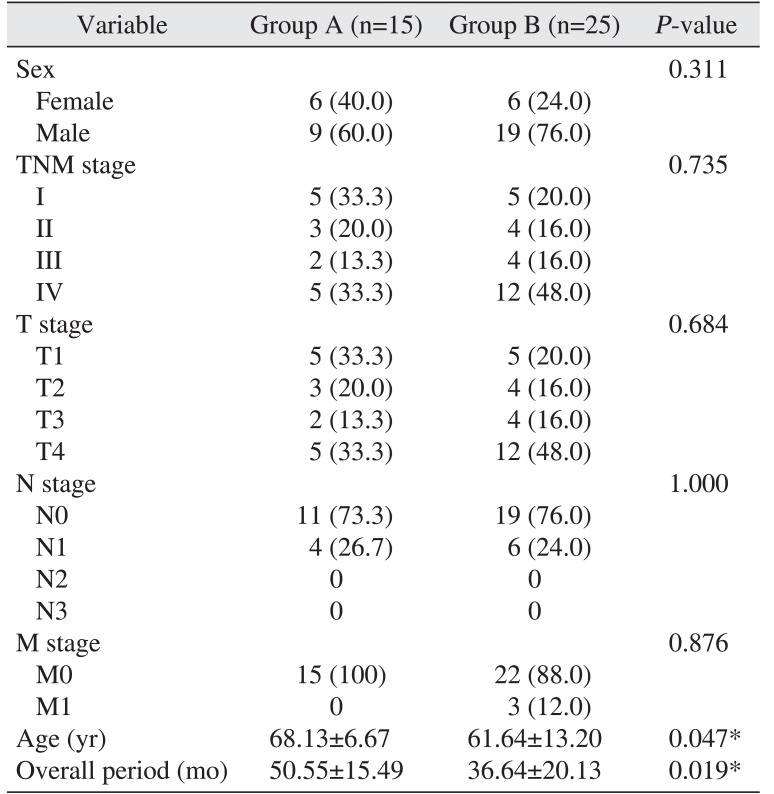
Table 2
Serological characteristic between COP-MPV groups A and B
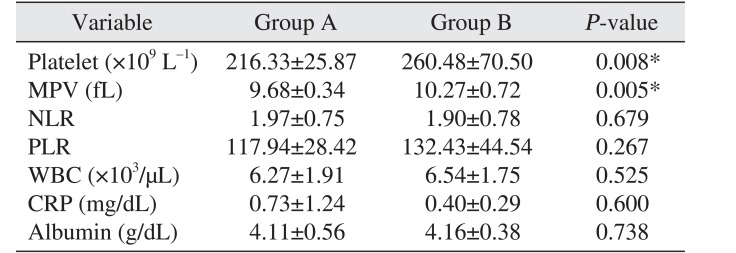
Table 3
Univariate and multivariate analyses of overall survival for oral squamous cell carcinoma patients




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download