This article has been
cited by other articles in ScienceCentral.
Abstract
Objectives
To test the feasibility of submandibular salivary gland (SMG) replantation techniques and the survival of the replanted glands. Such a study can provide a rationale for later allotransplantation procedures, along with implementation of conventional and advanced immunosuppression therapy.
Materials and Methods
Six SMG replantations were performed in New Zealand white rabbits. One week postoperatively, 99mTc scintigraphy was performed and the uptake ratio and salivary excretion fraction were calculated. Two to four weeks later, submandibular glands were excised, fixed, and stained with H&E for histomorphometric evaluation.
Results
Intraoperatively, all glands showed patent blood perfusion except gland 5. Positive tracer uptake and saliva excretion were documented by scintigraphy. On excision, all of the glands except glands 4 and 5 looked viable, with a red color and patent pedicles. Gland 4 was infected and filled with creamy pus, while gland 5 looked pale and necrotic. Histologically, glands 1, 2, 3, and 6 had preserved normal glandular tissue with slight variations from the contralateral normal glands, as their parenchyma was composed of mildly atrophic acini.
Conclusion
Four out of six replanted SMGs successfully survived. The glands maintained good viability and function. Such success depends on safe harvesting, short anastomosis time, and strict control of infection.
Go to :

Keywords: Rabbits, Submandibular gland, Replantation
I. Introduction
Xerostomia is a debilitating consequence of radiotherapy or systemic diseases like Sjögren's syndrome that affects quality of life. Saliva deficiency predisposes patients to infection, caries, periodontal disease, oral discomfort, altered taste, and difficulty in speaking, chewing and swallowing
12. Current treatments composed mainly of artificial saliva and/or systemic therapy like pilocarpine. Such treatments are mainly symptomatic but not curative
2. Establishment of submandibular salivary gland (SMG) transplantation seems to offer a promising treatment modality for both xerophthalmia and xerostomia. For the treatment of xerophthalmia, the SMG was transferred to the temporal fossa and saliva was drained into the conjunctival fornix to provide lubrication and prevent the development of corneal ulceration
3. Two-stage autotransplantation is another approach that has been employed to protect the gland from being exposed to harmful radiation. In the first stage, the gland is transferred to a radiation-free body structure such as the groin, and later the gland is dissected and transferred back to the neck to be replanted
4. However, damage to SMGs during the second exploration or failure of replantation has been reported
4. In addition, some individuals may have their submandibular glands missing or functionally compromised due to a generalized immunological disorder or postoperative irradiation. Allotransplantation of SMGs may be an alternative option for such situations.
The purpose of our study was to explore SMG transplantation by performing gland replantation in rabbits, where we tested the feasibility of the technique and survival of the replanted glands. This study may provide a rationale for later allotransplantation procedures, along with implementation of conventional and advanced immunosuppression therapy.
Go to :

II. Materials and Methods
1. Animals
Six SMG replantations were performed in six New Zealand white male rabbits, each rabbit weighed 3.0 to 3.5 kg. Left-sided glands were replanted while the right side acted as the control. All animals were followed for 2 to 4 weeks. All animal surgeries and experimental procedures were carried out in accordance with the care guidelines of the laboratory animal resources of Seoul National University Institutional Animal Care and Use Committee (IACUC), Republic of Korea (approval no. SNU-160720-6-1). The animals were caged individually, and their environment was maintained with a 12-hour light-dark cycle. Water and standard laboratory food were provided ad libitum.
2. Surgical procedure
Ketamine (35 mg/kg) and xylazine (5 mg/kg) were injected intramuscularly for anesthesia induction. Intravenous levofloxacin (5 mg/kg) and warm normal saline solution (2 mL/kg/h) were infused to prevent infection and dehydration. Anesthesia was maintained with isoflurane anesthetic (1.5%–2.0%) via a rabbit supraglottic V-gel tube (Docsinnovent, London, UK). After applying iodine solution to the surgical site, 0.9 mL of 2% lidocaine with epinephrine was injected subcutaneously. A midline skin incision was then made from the lower margin of the mandible to the level of the cricoid cartilage, followed by subplatysma muscle dissection to identify the linguofacial vein. The SMG was then dissected and separated from the tail of the parotid gland laterally and from the strap muscles medially. Care was taken to prevent gland capsule separation or glandular vessel mishandling. Through retraction of the sternomastoid and sternocephalic muscles, the common carotid artery trunk came into sight. Then, we severed the posterior belly of the digastric and stylohyoid muscles to manipulate the facial artery and its glandular branch. All of the common carotid artery branches were cut and ligated while preserving the facial artery and its glandular branch. Lingual and facial veins were ligated while preserving the linguofacial vein trunk and the glandular vein. Wharton's duct was identified anteromedially to the gland and dissected free until the point where it penetrated the floor of the mouth. In the first three gland replantations, the duct was kept intact so that the gland could drain intraorally. For the last three replantations, a 0.5-mm wide silicon tube (Beaver-Visitec, Waltham, MA, USA) was inserted into the duct and anchored to the neck skin. Proper intubation was achieved through tension-free orientation of the duct, longitudinal incision, and mild dilation.(
Fig. 1) The pedicle's common carotid artery and linguofacial vein were then cut and the gland was reflected back over the mandible and irrigated with warm normal saline for 10 minutes. Afterward, it was inset back to its original site and the common carotid artery and linguofacial vein were anastomosed to their proximal ends in end-to-end mode using 10/0 nylon (Ethicon, Livingston, UK) under a surgical microscope (Carl Zeiss, Oberkochen, Germany). The gland was then checked for the presence of proper blood perfusion and patent pedicle vessels. The wound was then irrigated gently with normal saline and closed using 4/0 Vicryl (Ethicon) for the platysma-fascia layer and 4/0 Dafilon (Braun, Barcelona, Spain) for the skin. A Penrose drain was placed for 3 days.
 | Fig. 1A. Intraoperative photo showing the replanted gland (white arrow), 0.5 mm tube cannulated into Wharton's duct (black arrow), anastomosed linguofacial vein (arrowhead), and common carotid artery (unviewed, located medially). B. The replanted gland 3 showing anastomosed common carotid artery (arrow) and anastomosis of the glandular vein to the linguofacial vein.
|
Postoperative care was carried out using levofloxacin (5 mg/kg) every 24 hours for 5 days and tramadol (5 mg/kg) twice a day for 3 days. Dextrose 5% (16 mL) and 0.9% normal saline (32 mL) were adjunctively supplied daily for 3 days. Assisted oral supply with Critical Care Formula (Oxbow, Murdock, NE, USA) was commenced on the 3rd postoperative day in case the rabbit did not resume its spontaneous feeding.
3. Surgical course and outcome
Wounds were assessed daily during the follow-up period for any swelling or pus discharge originating from the glands. At time of excision, glands were grossly evaluated for viability and pedicle patency.
4. Scintigraphy
One week postoperatively,
99mTc scintigraphy was performed using a gamma camera as mentioned previously by Hakim et al.
5 and Liu et al.
6. Briefly, after intravenous injection of 100 MBq
99mTcO
4−, the rabbits underwent sequential scintigraphy in the prone position and underwent basal projection of the head using a γ-camera (field-of-view, 25 cm); 0.01 mg/kg carbachol was injected subcutaneously at the 20th minute and scintigraphy was continued for another 25 minutes. Ninety frames were acquired with a frame per 30 seconds in a 256×256 matrix with a zoom of 4. Regions of interest (ROIs) were drawn by overlaying all frames of replanted glands and the contralateral glands. The background ROI was drawn laterally and caudally to the submandibular gland position. Dynamic time-activity curves were generated automatically for all drawn ROIs. Then, the uptake ratio (UR) and salivary excretion fraction (SEF) were calculated. UR was defined as the ratio between maximum radiation counts and the background radiation counts. The SEF was defined as the ratio of the difference between the maximum and minimum radiation counts to the maximum radiation counts.
UR=maximum radiation counts (≤20 minutes)/background radiation counts
SEF %=maximum radiation counts (≤20 minutes)−minimum radiation counts/maximum radiation counts
5. Histomorphometric analysis
At the end of the follow-up period, the replanted and contralateral glands were excised and fixed in 10% formaldehyde. Glands were then sectioned vertically into four equal specimens from base to apex. Specimens were reversed horizontally and embedded in paraffin, sectioned, and stained with hematoxylin-eosin for histomorphometric evaluation.
6. Statistical analysis
Data analysis was carried out using StatView software ver. 5.0.1 (SAS Institute, Cary, NC, USA). All data are presented as mean with standard error of the mean.
Go to :

III. Results
1. Surgical course and outcome
All replantation procedures were carried out by the same surgeon (A.A.A.). Six glands were replanted by anastomosing the linguofacial vein and common carotid artery to their corresponding proximal ends in an end-to-end mode. In gland 3, the distal linguofacial vein was damaged during cauterization of the facial vein, so the glandular vein was directly anastomosed to the proximal linguofacial vein. After replantation, the glands exhibited proper blood perfusion and patent pedicles except gland 5, where the venous blood was alternately flowing slowly and in small amounts. The skin-anchored tubes, which were placed for the last three rabbits, were removed by the rabbits postoperatively, which precluded the measurement of saliva flow rate. The rabbits were kept for 2 to 4 weeks until gland excision. Rabbits 1 to 4 were kept for 4 weeks, while rabbits 5 and 6 were kept for 2 weeks.(
Table 1)
Table 1
Rabbit surgical courses and outcomes
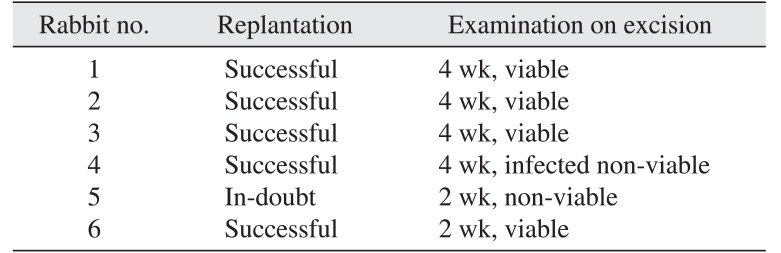
|
Rabbit no. |
Replantation |
Examination on excision |
|
1 |
Successful |
4 wk, viable |
|
2 |
Successful |
4 wk, viable |
|
3 |
Successful |
4 wk, viable |
|
4 |
Successful |
4 wk, infected non-viable |
|
5 |
In-doubt |
2 wk, non-viable |
|
6 |
Successful |
2 wk, viable |

2. Sialoscintigraphy
The presence of summative uptake of adjacent glands could prevent the evaluation of SMGs in rabbits. However, in the current study, the replanted glands exhibited higher uptake of the tracer, which enabled us to distinguish them from their surroundings.(
Fig. 2) The scintigraphy revealed a positive UR for the replanted glands except gland 5, with a mean of 3.0±0.9, while the contralateral normal glands had a mean of 2.6±1.1. The SEF was similar to that of contralateral normal glands, with a mean value of 25.7% and 25.1%, respectively. (
Table 2)
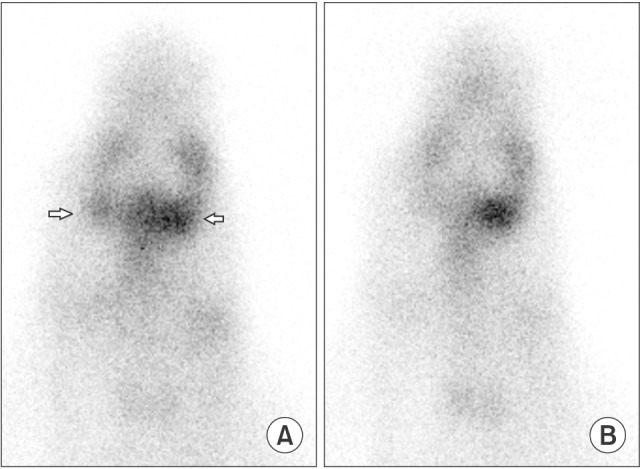 | Fig. 2A. Summit sialoscintigraphy (15th to 20th minute) of rabbit 2 showing higher uptake of tracer in the replanted gland left side in compare to the contralateral normal right side (arrows). B. Postcarbachol-injection sialoscintigraphy (40th to 45th minute) where both glands exhibited tracer excretion.
|
Table 2
Uptake ratio (UR) and salivary ejection fraction (SEF) for the replanted and normal contralateral glands as revealed by sialoscintigraphy
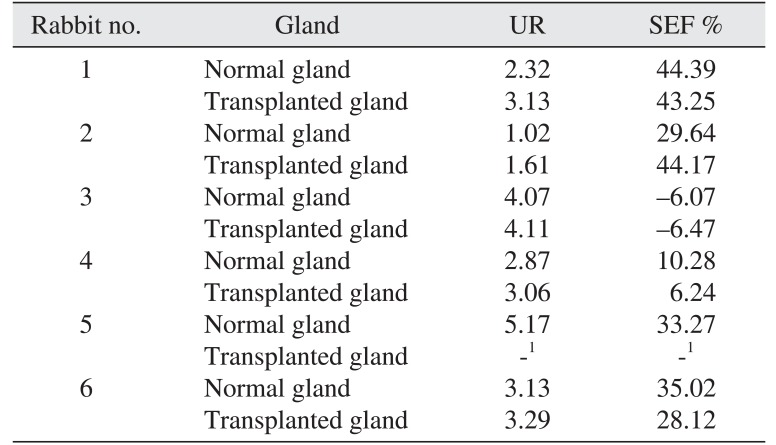
|
Rabbit no. |
Gland |
UR |
SEF % |
|
1 |
Normal gland |
2.32 |
44.39 |
|
Transplanted gland |
3.13 |
43.25 |
|
2 |
Normal gland |
1.02 |
29.64 |
|
Transplanted gland |
1.61 |
44.17 |
|
3 |
Normal gland |
4.07 |
−6.07 |
|
Transplanted gland |
4.11 |
−6.47 |
|
4 |
Normal gland |
2.87 |
10.28 |
|
Transplanted gland |
3.06 |
6.24 |
|
5 |
Normal gland |
5.17 |
33.27 |
|
Transplanted gland |
-1
|
-1
|
|
6 |
Normal gland |
3.13 |
35.02 |
|
Transplanted gland |
3.29 |
28.12 |

3. Histomorphometric analysis
At the end of the follow-up period, the neck wound was reopened and the replanted glands were evaluated grossly. All of the glands except glands 4 and 5 looked viable, with red color and patent pedicles. Gland 4 was infected and filled with creamy pus while gland 5 looked pale and necrotic.
Histologically, glands 1, 2, 3, and 6 had preserved normal glandular tissue with slight variation from the contralateral glands, as their parenchyma was composed of mildly atrophic acini. Glands 3 and 6 were occupied by frequent ducts like the contralateral normal glands, while glands 1 and 2 had fewer ducts in their parenchyma.(
Fig. 3. A, 3. B)
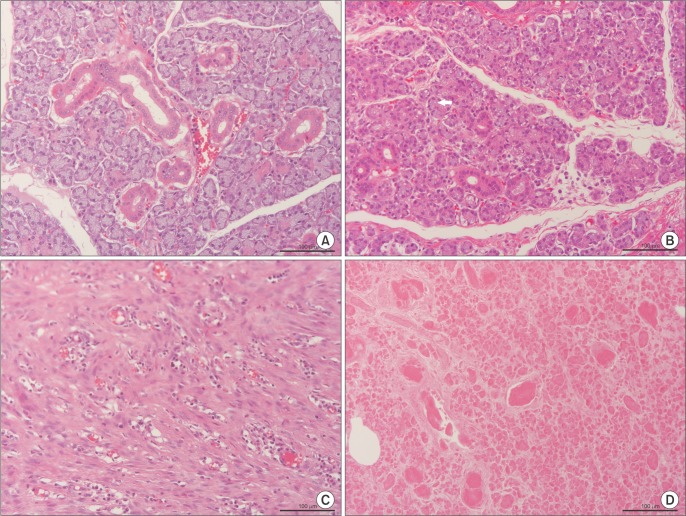 | Fig. 3A. Histologic structure of a control submandibular gland with intact acini and ducts (H&E staining, ×20; scale bar=100 µm). B. Histologic
structure of the viable replanted gland 6 with mild atrophy of acinar cells (arrow) (H&E staining, ×20; scale bar=100 µm). C. Histologic
structure of the infected-nonviable replanted gland 4 with cords of connective tissue, inflammatory cell invasion, and total disappearance
of acini (H&E staining, ×20; scale bar=100 µm). D. Histologic structure of the necrotic replanted gland 5 with degeneration of acini (H&E
staining, ×20; scale bar=100 µm).
|
Because gland 4 was infected, it was invaded by many inflammatory cells, which were mostly lymphocytes.(
Fig. 3. C) Degenerated acini and fibrous tissue filled with multiple thrombotic foci were observed in gland 5; this histological picture reflects the lack of blood perfusion and necrosis.(
Fig. 3. D)
Go to :

IV. Discussion
The success rate of autologous transplanted SMGs in rabbit animal models was reported as 15 viable glands out of 20 in a study by Kumar et al.
3 and 4 viable transplanted glands out of 6 glands in a study by Spiegel et al.
4. In Su et al.
7, 4 glands out of 5 in a dog model were reported as viable with no sialocele formation.
Success in SMG transplantation requires safe harvesting to avoid injury to the small glandular vessels. In gland 5, damaging the glandular artery branch while dividing and cauterizing the adjacent vessels caused its failure. Gland 4 was infected and filled with creamy pus. Encapsulated creamy pus was also found in rabbits 1 and 2, but far away from the replanted glands. Such complications should be properly addressed in future procedures through vigorous saline irrigation of the wound, daily dressing, and proper postoperative antibiotic treatment. Quick and accurate microanastomosis is another outstanding factor. In our study, we used the linguofacial vein and common carotid artery as pedicles, and both of them measured around 1.5 to 2.0 mm. This size enabled very convenient anastomosis. In rabbits, the common carotid artery gives off its final branches at the level of the hyoid bone. The distance between the internal carotid artery and the trifurcation of the external carotid artery is less than 10 mm, which precludes the use of the external carotid artery as the distal end of the pedicle
8. The internal jugular vein is a small vein that branches a few millimeters above the clavicle, while the external jugular vein is a large vein that gives off two main branches; the maxillary vein and the linguofacial vein. The linguofacial vein has three branches; the glandular vein, the facial vein, and the lingual vein.
Sieg et al.
9 evaluated the effect of ischemia duration on the viability of submandibular glands by interrupting blood flow for 1.0, 1.5, 2.0, and 6.0 hours. Increasing structural damage of the parenchyma began at 1.5 hours of ischemia. The cell proliferation in the remaining salivary gland tissue after 1.5 or 2.0 hours of ischemia was significantly increased. Six hours of ischemia led to total necrosis of the salivary glands.
In a study by Kumar et al.
3, where the SMG was transferred to the temporal region; saliva flow drainage was performed by making a tunnel in the upper conjunctival fornix for Wharton duct passage. Measurement was carried out by placement of a small filter paper into the lower conjunctival fornix. It was not possible to perform duct cannulation in two studies dealing with SMG replantation in the neck area of rabbit and dog animal models. Spiegel et al.
4 placed the free end of the duct next to the gland and evaluated salivary gland function by examining the presence of sialocele at time of gland excision. Su et al.
7 stated that it was difficult to restore drainage by suturing the gland duct to the external skin in a dog due to its small size. In our study, we intubated the duct using a 0.5 mm silicon tube and fixed it to the neck skin for the last three rabbits, but the rabbits removed the tubes as they were accessible and there was only weak fixation (using a 9/0 suture) between the duct and the tiny artificial tube or between the tube and skin.
Sialoscintigraphy is an alternative non-invasive method to evaluate both viability and function of the gland. Unlike previous reports which individually evaluated SMG in rabbits
1011, Hakim et al.
12 clarified the difficulty in selectively imaging the SMG, due to the merge effect for tracer uptake in lingual, buccal, and pharyngeal glands compared with submandibular glands. In our study, replanted submandibular glands showed more enhancement and uptake of the tracer, which enabled us to differentiate them from adjacent glands. This could be due to the increase in vascularity and/or inflammatory reaction within the replanted glands in the early postoperative period. In the literature, saliva hypersecretion was reported in the early days after transplantation, and release of neurotransmitters from degenerating sympathetic terminal axons may account for this phenomenon
13. For further studies, we would recommend modifying the gland location by around 1 cm lateral and caudal to their original position to avoid any summating effect from surrounding glands.
The histological structure of autologous transplanted salivary glands ranged from mild atrophy in the parenchyma to total necrosis. Kumar et al.
3 showed that histological examination of the transferred gland revealed normal structure in 8 glands, while more than 50% of the gland was normal in 7 more glands. Two glands were completely necrotic and in three others, more than 50% of the gland was replaced by fibrous tissue or fat. Spiegel et al.
4 showed two preserved glands with normal gland architecture, two glands with mild inflammation, acinar loss and vacuolization, and one gland with marked inflammatory infiltrate and necrotic acini. Su et al.
7 reported the presence of numerous lymphocytes around the ducts and within the parenchyma, abundant macrophages, and severe fibrosis with final autolysis in the replanted glands.
Go to :

V. Conclusion
Four out of six replanted SMGs successfully survived. The glands maintained good viability and function. Such success depends on safe harvesting, quick anastomosis, and strict control of infection.
Go to :









 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download