1. Gadre KS, Halli R, Joshi S, Ramanojam S, Gadre PK, Kunchur R, et al. Incidence and pattern of cranio-maxillofacial injuries: a 22 year retrospective analysis of cases operated at major trauma hospitals/centres in Pune, India. J Maxillofac Oral Surg. 2013; 12:372–378. PMID:
24431873.
3. Gassner R, Tuli T, Hächl O, Rudisch A, Ulmer H. Cranio-maxillofacial trauma: a 10 year review of 9,543 cases with 21,067 injuries. J Craniomaxillofac Surg. 2003; 31:51–61. PMID:
12553928.

4. Hikita A, Chung UI, Hoshi K, Takato T. Bone regenerative medicine in oral and maxillofacial region using a three-dimensional printer. Tissue Eng Part A. 2017; 23:515–521. PMID:
28351222.

5. Lew TA, Walker JA, Wenke JC, Blackbourne LH, Hale RG. Characterization of craniomaxillofacial battle injuries sustained by United States service members in the current conflicts of Iraq and Afghanistan. J Oral Maxillofac Surg. 2010; 68:3–7. PMID:
20006147.

6. Hale RG, Lew T, Wenke JC. Craniomaxillofacial battle injuries: injury patterns, conventional treatment limitations and direction of future research. Singapore Dent J. 2010; 31:1–8. PMID:
23739250.

7. Chan RK, Siller-Jackson A, Verrett AJ, Wu J, Hale RG. Ten years of war: a characterization of craniomaxillofacial injuries incurred during operations Enduring Freedom and Iraqi Freedom. J Trauma Acute Care Surg. 2012; 73(6 Suppl 5):S453–S458. PMID:
23192069.
8. Brown Baer PR, Wenke JC, Thomas SJ, Hale CR. Investigation of severe craniomaxillofacial battle injuries sustained by U.S. service members: a case series. Craniomaxillofac Trauma Reconstr. 2012; 5:243–252. PMID:
24294409.

9. Carano RA, Filvaroff EH. Angiogenesis and bone repair. Drug Discov Today. 2003; 8:980–989. PMID:
14643161.

10. Alsberg E, Hill EE, Mooney DJ. Craniofacial tissue engineering. Crit Rev Oral Biol Med. 2001; 12:64–75. PMID:
11349963.

11. Kraft A, Abermann E, Stigler R, Zsifkovits C, Pedross F, Kloss F, et al. Craniomaxillofacial trauma: synopsis of 14,654 cases with 35,129 injuries in 15 years. Craniomaxillofac Trauma Reconstr. 2012; 5:41–50. PMID:
23449961.

12. Bose S, Vahabzadeh S, Bandyopadhyay A. Bone tissue engineering using 3D printing. Materials Today. 2013; 16:496–504.

13. Mehta M, Schmidt-Bleek K, Duda GN, Mooney DJ. Biomaterial delivery of morphogens to mimic the natural healing cascade in bone. Adv Drug Deliv Rev. 2012; 64:1257–1276. PMID:
22626978.

14. Klammert U, Gbureck U, Vorndran E, Rödiger J, Meyer-Marcotty P, Kübler AC. 3D powder printed calcium phosphate implants for reconstruction of cranial and maxillofacial defects. J Craniomaxillofac Surg. 2010; 38:565–570. PMID:
20206538.

15. Parthasarathy J. 3D modeling, custom implants and its future perspectives in craniofacial surgery. Ann Maxillofac Surg. 2014; 4:9–18. PMID:
24987592.

16. Bergmann C, Lindner M, Zhang W, Koczur K, Kirsten A, Telle R, et al. 3D printing of bone substitute implants using calcium phosphate and bioactive glasses. J Eur Ceram Soc. 2010; 30:2563–2567.

17. Inzana JA, Olvera D, Fuller SM, Kelly JP, Graeve OA, Schwarz EM, et al. 3D printing of composite calcium phosphate and collagen scaffolds for bone regeneration. Biomaterials. 2014; 35:4026–4034. PMID:
24529628.

18. Cohen A, Laviv A, Berman P, Nashef R, Abu-Tair J. Mandibular reconstruction using stereolithographic 3-dimensional printing modeling technology. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009; 108:661–666. PMID:
19716728.

19. Gross BC, Erkal JL, Lockwood SY, Chen C, Spence DM. Evaluation of 3D printing and its potential impact on biotechnology and the chemical sciences. Anal Chem. 2014; 86:3240–3253. PMID:
24432804.

20. Farré-Guasch E, Wolff J, Helder MN, Schulten EA, Forouzanfar T, Klein-Nulend J. Application of additive manufacturing in oral and maxillofacial surgery. J Oral Maxillofac Surg. 2015; 73:2408–2418. PMID:
25966454.

21. Xiao K, Zardawi F, van Noort R, Yates JM. Developing a 3D colour image reproduction system for additive manufacturing of facial prostheses. Int J Adv Manuf Technol. 2014; 70:2043–2049.

22. Butscher A, Bohner M, Hofmann S, Gauckler L, Müller R. Structural and material approaches to bone tissue engineering in powder-based three-dimensional printing. Acta Biomater. 2011; 7:907–920. PMID:
20920616.

23. Chia HN, Wu BM. Recent advances in 3D printing of biomaterials. J Biol Eng. 2015; 9:4. PMID:
25866560.

24. Guvendiren M, Molde J, Soares RM, Kohn J. Designing biomaterials for 3D printing. ACS Biomater Sci Eng. 2016; 2:1679–1693. PMID:
28025653.

25. Liu YF, Xu LW, Zhu HY, Liu SS. Technical procedures for template-guided surgery for mandibular reconstruction based on digital design and manufacturing. Biomed Eng Online. 2014; 13:63. PMID:
24886431.

26. Schwam ZG, Chang MT, Barnes MA, Paskhover B. Applications of 3-dimensional printing in facial plastic surgery. J Oral Maxillofac Surg. 2016; 74:427–428. PMID:
26611375.

27. Dawood A, Marti Marti B, Sauret-Jackson V, Darwood A. 3D printing in dentistry. Br Dent J. 2015; 219:521–529. PMID:
26657435.

28. Malik HH, Darwood AR, Shaunak S, Kulatilake P, El-Hilly AA, Mulki O, et al. Three-dimensional printing in surgery: a review of current surgical applications. J Surg Res. 2015; 199:512–522. PMID:
26255224.

29. Rengier F, Mehndiratta A, von Tengg-Kobligk H, Zechmann CM, Unterhinninghofen R, Kauczor HU, et al. 3D printing based on imaging data: review of medical applications. Int J Comput Assist Radiol Surg. 2010; 5:335–341. PMID:
20467825.

30. Cillo JE Jr, Basi D, Peacock Z, Aghaloo T, Bouloux G, Dodson T, et al. Proceedings of the American Association of Oral and Maxillofacial Surgeons 2015 Research Summit. J Oral Maxillofac Surg. 2016; 74:429–437. PMID:
26707430.

31. Velasco I, Vahdani S, Ramos H, Guzman J. Clinical application of desktop three-dimensional printing technology in ablative and reconstructive maxillofacial surgery. Oral Surg Oral Med Oral Pathol Oral Radiol. 2017; 123:e23–e24.

32. Thomas DJ, Azmi MABM, Tehrani Z. 3D additive manufacture of oral and maxillofacial surgical models for preoperative planning. Int J Adv Manuf Technol. 2014; 71:1643–1651.

33. Leukers B, Gülkan H, Irsen SH, Milz S, Tille C, Schieker M, et al. Hydroxyapatite scaffolds for bone tissue engineering made by 3D printing. J Mater Sci Mater Med. 2005; 16:1121–1124. PMID:
16362210.

34. Khalyfa A, Vogt S, Weisser J, Grimm G, Rechtenbach A, Meyer W, et al. Development of a new calcium phosphate powder-binder system for the 3D printing of patient specific implants. J Mater Sci Mater Med. 2007; 18:909–916. PMID:
17216579.

35. Zhou Z, Buchanan F, Mitchell C, Dunne N. Printability of calcium phosphate: calcium sulfate powders for the application of tissue engineered bone scaffolds using the 3D printing technique. Mater Sci Eng C Mater Biol Appl. 2014; 38:1–10. PMID:
24656346.

36. Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005; 366:1809–1820. PMID:
16298220.

37. Eke PI, Dye BA, Wei L, Thornton-Evans GO, Genco RJ. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J Dent Res. 2012; 91:914–920. PMID:
22935673.

38. Wang J, Zhang R, Shen Y, Xu C, Qi S, Lu L, et al. Recent advances in cell sheet technology for periodontal regeneration. Curr Stem Cell Res Ther. 2014; 9:162–173. PMID:
24524797.

39. Tatullo M, Marrelli M, Shakesheff KM, White LJ. Dental pulp stem cells: function, isolation and applications in regenerative medicine. J Tissue Eng Regen Med. 2015; 9:1205–1216. PMID:
24850632.

40. Nanci A, Bosshardt DD. Structure of periodontal tissues in health and disease. Periodontol 2000. 2006; 40:11–28. PMID:
16398683.

41. Kim JH, Park CH, Perez RA, Lee HY, Jang JH, Lee HH, et al. Advanced biomatrix designs for regenerative therapy of periodontal tissues. J Dent Res. 2014; 93:1203–1211. PMID:
25139364.

42. Ivanovski S, Vaquette C, Gronthos S, Hutmacher DW, Bartold PM. Multiphasic scaffolds for periodontal tissue engineering. J Dent Res. 2014; 93:1212–1221. PMID:
25139362.

43. Jakab K, Norotte C, Marga F, Murphy K, Vunjak-Novakovic G, Forgacs G. Tissue engineering by self-assembly and bio-printing of living cells. Biofabrication. 2010; 2:022001. PMID:
20811127.

44. Chen YW, Hsu TT, Wang K, Shie MY. Preparation of the fast setting and degrading Ca-Si-Mg cement with both odontogenesis and angiogenesis differentiation of human periodontal ligament cells. Mater Sci Eng C Mater Biol Appl. 2016; 60:374–383. PMID:
26706543.

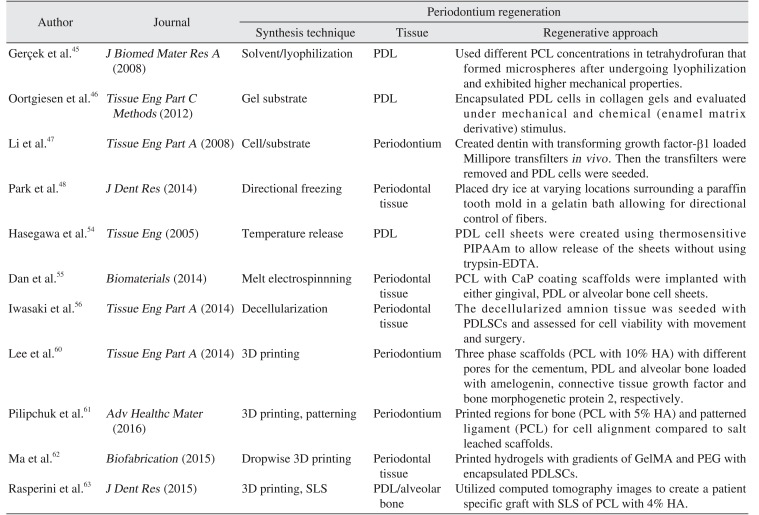
45. Gerçek I, Tigli RS, Gümüşderelioglu M. A novel scaffold based on formation and agglomeration of PCL microbeads by freeze-drying. J Biomed Mater Res A. 2008; 86:1012–1022. PMID:
18067167.

46. Oortgiesen DA, Yu N, Bronckers AL, Yang F, Walboomers XF, Jansen JA. A three-dimensional cell culture model to study the mechano-biological behavior in periodontal ligament regeneration. Tissue Eng Part C Methods. 2012; 18:81–89. PMID:
21913838.

47. Li Y, Jin F, Du Y, Ma Z, Li F, Wu G, et al. Cementum and periodontal ligament-like tissue formation induced using bioengineered dentin. Tissue Eng Part A. 2008; 14:1731–1742. PMID:
18636796.

48. Park CH, Kim KH, Rios HF, Lee YM, Giannobile WV, Seol YJ. Spatiotemporally controlled microchannels of periodontal mimic scaffolds. J Dent Res. 2014; 93:1304–1312. PMID:
25216511.

49. Eleuterio E, Trubiani O, Sulpizio M, Di Giuseppe F, Pierdomenico L, Marchisio M, et al. Proteome of human stem cells from periodontal ligament and dental pulp. PLoS One. 2013; 8:e71101. PMID:
23940696.

50. Horst OV, Chavez MG, Jheon AH, Desai T, Klein OD. Stem cell and biomaterials research in dental tissue engineering and regeneration. Dent Clin North Am. 2012; 56:495–520. PMID:
22835534.

51. Liu B, Song YW, Jin L, Wang ZJ, Pu DY, Lin SQ, et al. Silk structure and degradation. Colloids Surf B Biointerfaces. 2015; 131:122–128. PMID:
25982316.

52. Yeasmin S, Ceccarelli J, Vigen M, Carrion B, Putnam AJ, Tarle SA, et al. Stem cells derived from tooth periodontal ligament enhance functional angiogenesis by endothelial cells. Tissue Eng Part A. 2014; 20:1188–1196. PMID:
24147894.

53. Alves LB, Mariguela VC, Grisi MF, Souza SL, Novaes Junior AB, Taba Junior M, et al. Expression of osteoblastic phenotype in periodontal ligament fibroblasts cultured in three-dimensional collagen gel. J Appl Oral Sci. 2015; 23:206–214. PMID:
26018313.

54. Hasegawa M, Yamato M, Kikuchi A, Okano T, Ishikawa I. Human periodontal ligament cell sheets can regenerate periodontal ligament tissue in an athymic rat model. Tissue Eng. 2005; 11:469–478. PMID:
15869425.

55. Dan H, Vaquette C, Fisher AG, Hamlet SM, Xiao Y, Hutmacher DW, et al. The influence of cellular source on periodontal regeneration using calcium phosphate coated polycaprolactone scaffold supported cell sheets. Biomaterials. 2014; 35:113–122. PMID:
24120045.

56. Iwasaki K, Komaki M, Yokoyama N, Tanaka Y, Taki A, Honda I, et al. Periodontal regeneration using periodontal ligament stem cell-transferred amnion. Tissue Eng Part A. 2014; 20:693–704. PMID:
24032400.

57. Li X, Cui R, Sun L, Aifantis KE, Fan Y, Feng Q, et al. 3D-printed biopolymers for tissue engineering application. Int J Polym Sci. 2014; 2014:1–13.

58. Li J, He L, Zhou C, Zhou Y, Bai Y, Lee FY, et al. 3D printing for regenerative medicine: from bench to bedside. MRS Bull. 2015; 40:145–154.

59. Kim K, Lee CH, Kim BK, Mao JJ. Anatomically shaped tooth and periodontal regeneration by cell homing. J Dent Res. 2010; 89:842–847. PMID:
20448245.

60. Lee CH, Hajibandeh J, Suzuki T, Fan A, Shang P, Mao JJ. Three-dimensional printed multiphase scaffolds for regeneration of periodontium complex. Tissue Eng Part A. 2014; 20:1342–1351. PMID:
24295512.

61. Pilipchuk SP, Monje A, Jiao Y, Hao J, Kruger L, Flanagan CL, et al. Integration of 3D printed and micropatterned polycaprolactone scaffolds for guidance of oriented collagenous tissue formation in vivo. Adv Healthc Mater. 2016; 5:676–687. PMID:
26820240.

62. Ma Y, Ji Y, Huang G, Ling K, Zhang X, Xu F. Bioprinting 3D cell-laden hydrogel microarray for screening human periodontal ligament stem cell response to extracellular matrix. Biofabrication. 2015; 7:044105. PMID:
26696269.

63. Rasperini G, Pilipchuk SP, Flanagan CL, Park CH, Pagni G, Hollister SJ, et al. 3D-printed bioresorbable scaffold for periodontal repair. J Dent Res. 2015; 94(9 Suppl):153S–157S. PMID:
26124215.

64. Yildirim S, Fu SY, Kim K, Zhou H, Lee CH, Li A, et al. Tooth regeneration: a revolution in stomatology and evolution in regenerative medicine. Int J Oral Sci. 2011; 3:107–116. PMID:
21789959.

65. Kwak SY, Litman A, Margolis HC, Yamakoshi Y, Simmer JP. Biomimetic enamel regeneration mediated by leucine-rich amelogenin peptide. J Dent Res. 2017; 96:524–530. PMID:
28113034.

66. Siamos G, Winkler S, Boberick KG. Relationship between implant preload and screw loosening on implant-supported prostheses. J Oral Implantol. 2002; 28:67–73. PMID:
12498448.
67. Cross D, El-Angbawi A, McLaughlin P, Keightley A, Brocklebank L, Whitters J, et al. Developments in autotransplantation of teeth. Surgeon. 2013; 11:49–55. PMID:
23142342.

68. Kato A, Ohno N. Construction of three-dimensional tooth model by micro-computed tomography and application for data sharing. Clin Oral Investig. 2009; 13:43–46.

69. Li J, Zhang L, Lv S, Li S, Wang N, Zhang Z. Fabrication of individual scaffolds based on a patient-specific alveolar bone defect model. J Biotechnol. 2011; 151:87–93. PMID:
21056602.

70. Bose S, Darsell J, Hosick HL, Yang L, Sarkar DK, Bandyopadhyay A. Processing and characterization of porous alumina scaffolds. J Mater Sci Mater Med. 2002; 13:23–28. PMID:
15348200.
71. Sumita Y, Honda MJ, Ohara T, Tsuchiya S, Sagara H, Kagami H, et al. Performance of collagen sponge as a 3-D scaffold for tooth-tissue engineering. Biomaterials. 2006; 27:3238–3248. PMID:
16504285.

72. Honda MJ, Tsuchiya S, Sumita Y, Sagara H, Ueda M. The sequential seeding of epithelial and mesenchymal cells for tissue-engineered tooth regeneration. Biomaterials. 2007; 28:680–689. PMID:
17045644.

73. Sloan AJ, Rutherford RB, Smith AJ. Stimulation of the rat dentine-pulp complex by bone morphogenetic protein-7 in vitro. Arch Oral Biol. 2000; 45:173–177. PMID:
10716622.

74. Dobie K, Smith G, Sloan AJ, Smith AJ. Effects of alginate hydrogels and TGF-beta 1 on human dental pulp repair in vitro. Connect Tissue Res. 2002; 43:387–390. PMID:
12489186.
75. Kuo TF, Huang AT, Chang HH, Lin FH, Chen ST, Chen RS, et al. Regeneration of dentin-pulp complex with cementum and periodontal ligament formation using dental bud cells in gelatin-chondroitin-hyaluronan tri-copolymer scaffold in swine. J Biomed Mater Res A. 2008; 86:1062–1068. PMID:
18067171.

76. Young CS, Terada S, Vacanti JP, Honda M, Bartlett JD, Yelick PC. Tissue engineering of complex tooth structures on biodegradable polymer scaffolds. J Dent Res. 2002; 81:695–700. PMID:
12351668.

77. Duailibi MT, Duailibi SE, Young CS, Bartlett JD, Vacanti JP, Yelick PC. Bioengineered teeth from cultured rat tooth bud cells. J Dent Res. 2004; 83:523–528. PMID:
15218040.

78. Anitua E, Alkhraisat MH, Orive G. Perspectives and challenges in regenerative medicine using plasma rich in growth factors. J Control Release. 2012; 157:29–38. PMID:
21763737.

79. Iohara K, Nakashima M, Ito M, Ishikawa M, Nakasima A, Akamine A. Dentin regeneration by dental pulp stem cell therapy with recombinant human bone morphogenetic protein 2. J Dent Res. 2004; 83:590–595. PMID:
15271965.

80. Huang GT, Yamaza T, Shea LD, Djouad F, Kuhn NZ, Tuan RS, et al. Stem/progenitor cell-mediated de novo regeneration of dental pulp with newly deposited continuous layer of dentin in an in vivo model. Tissue Eng Part A. 2010; 16:605–615. PMID:
19737072.

81. Janjić K, Cvikl B, Moritz A, Agis H. Dental pulp regeneration. Int J Stomat Occ Med. 2016; 8(Suppl 1):1–9.

82. Guo W, He Y, Zhang X, Lu W, Wang C, Yu H, et al. The use of dentin matrix scaffold and dental follicle cells for dentin regeneration. Biomaterials. 2009; 30:6708–6723. PMID:
19767098.

83. Li R, Guo W, Yang B, Guo L, Sheng L, Chen G, et al. Human treated dentin matrix as a natural scaffold for complete human dentin tissue regeneration. Biomaterials. 2011; 32:4525–4538. PMID:
21458067.

84. Hu B, Nadiri A, Kuchler-Bopp S, Perrin-Schmitt F, Peters H, Lesot H. Tissue engineering of tooth crown, root, and periodontium. Tissue Eng. 2006; 12:2069–2075. PMID:
16968149.

85. Yang B, Chen G, Li J, Zou Q, Xie D, Chen Y, et al. Tooth root regeneration using dental follicle cell sheets in combination with a dentin matrix-based scaffold. Biomaterials. 2012; 33:2449–2461. PMID:
22192537.
86. Wei F, Song T, Ding G, Xu J, Liu Y, Liu D, et al. Functional tooth restoration by allogeneic mesenchymal stem cell-based bio-root regeneration in swine. Stem Cells Dev. 2013; 22:1752–1762. PMID:
23363023.

87. Rosa V, Zhang Z, Grande RH, Nör JE. Dental pulp tissue engineering in full-length human root canals. J Dent Res. 2013; 92:970–975. PMID:
24056227.

88. Guda T, Appleford M, Oh S, Ong JL. A cellular perspective to bioceramic scaffolds for bone tissue engineering: the state of the art. Curr Top Med Chem. 2008; 8:290–299. PMID:
18393892.

89. McMenamin PG, Quayle MR, McHenry CR, Adams JW. The production of anatomical teaching resources using three-dimensional (3D) printing technology. Anat Sci Educ. 2014; 7:479–486. PMID:
24976019.

90. Obregon F, Vaquette C, Ivanovski S, Hutmacher DW, Bertassoni LE. Three-dimensional bioprinting for regenerative dentistry and craniofacial tissue engineering. J Dent Res. 2015; 94(9 Suppl):143S–152S. PMID:
26124216.

91. Fielding GA, Bandyopadhyay A, Bose S. Effects of silica and zinc oxide doping on mechanical and biological properties of 3D printed tricalcium phosphate tissue engineering scaffolds. Dent Mater. 2012; 28:113–122. PMID:
22047943.

92. Vaquette C, Fan W, Xiao Y, Hamlet S, Hutmacher DW, Ivanovski S. A biphasic scaffold design combined with cell sheet technology for simultaneous regeneration of alveolar bone/periodontal ligament complex. Biomaterials. 2012; 33:5560–5573. PMID:
22575832.

93. Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007; 100:1249–1260. PMID:
17495232.

94. Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol. 2007; 213:341–347. PMID:
17620285.

95. Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007; 318:1917–1920. PMID:
18029452.

96. Yan M, Yu Y, Zhang G, Tang C, Yu J. A journey from dental pulp stem cells to a bio-tooth. Stem Cell Rev. 2011; 7:161–171. PMID:
20506048.

97. Otsu K, Kumakami-Sakano M, Fujiwara N, Kikuchi K, Keller L, Lesot H, et al. Stem cell sources for tooth regeneration: current status and future prospects. Front Physiol. 2014; 5:36. PMID:
24550845.

98. Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000; 97:13625–13630. PMID:
11087820.
99. Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, et al. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A. 2003; 100:5807–5812. PMID:
12716973.

100. Ma L, Makino Y, Yamaza H, Akiyama K, Hoshino Y, Song G, et al. Cryopreserved dental pulp tissues of exfoliated deciduous teeth is a feasible stem cell resource for regenerative medicine. PLoS One. 2012; 7:e51777. PMID:
23251621.

101. Wang X, Sha XJ, Li GH, Yang FS, Ji K, Wen LY, et al. Comparative characterization of stem cells from human exfoliated deciduous teeth and dental pulp stem cells. Arch Oral Biol. 2012; 57:1231–1240. PMID:
22455989.

102. Sonoyama W, Liu Y, Fang D, Yamaza T, Seo BM, Zhang C, et al. Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS One. 2006; 1:e79. PMID:
17183711.

103. de Wert G, Mummery C. Human embryonic stem cells: research, ethics and policy. Hum Reprod. 2003; 18:672–682. PMID:
12660256.
104. Michalski MH, Ross JS. The shape of things to come: 3D printing in medicine. JAMA. 2014; 312:2213–2214. PMID:
25461994.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download