Abstract
Bisphosphonates are drugs used to treat osteoclast-mediated bone resorption, including osteoporosis, Paget disease, multiple myeloma, cancer-related osteolysis, and malignant hypercalcemia. The use of these drugs has increased in recent years as have their complications, especially bisphosphonate-related osteonecrosis of the jaw (BRONJ), which more frequently affects the mandible. Here we report a case of BRONJ with a particularly unfavorable course due to cervical inflammation that developed into necrotizing fasciitis, followed by multiorgan involvement leading to septic shock and death.
Bisphosphonates (BP) are pyrophosphate-like molecules that have been used since the 1960s to prevent and treat osteoporosis. The new and more effective amino-substituted BP are mainly used to treat Paget's disease, multiple myeloma, bone metastases, and associated malignant hypercalcemia. Use of these drugs has progressively increased, explaining the higher incidence of associated complications.
The first side-effects of BP were described in 1996 by de Groen et al.1 and consisted of common ulceration of the esophagus on administration of alendronate. The most common side-effect of these new drugs is bisphosphonate-related osteonecrosis of the jaw (BRONJ), which more frequently affects the mandible, presumably favored by the particular vascularization of this bone segment. BRONJ was first described by Marx2 in the early 2000s and is currently diagnosed on the basis of necrotic bone exposed in the oral cavity for at least 8 weeks in patients with no history of head or neck radiotherapy34.
Exposed necrotic bone is the distinctive sign of the disease, but the clinical picture can be varied and heterogeneous, including jaw pain, swelling, abscesses, and fistulas (sometimes cutaneous)5. Here we describe a case of BRONJ characterized by a particularly unfavorable course due to cervical inflammation that degenerated into necrotizing fasciitis with multiorgan involvement, followed by septic shock and death.
A 69-year-old female patient was treated at our department for osteonecrosis involving the upper and lower jaw, diagnosed and confirmed by histological examination in December 2015. She had undergone surgery for breast cancer on March 2015 and has been continuously treated with zoledronic acid since May 2015 (4 mg intravenous [i.v.] every 3 weeks). She also suffered from diabetes and atrial fibrillation. No dental events could explain the osteonecrosis.
The patient was treated for the necrosis (mechanical debridement, washing with hydrogen peroxide, iodopovidone, and 1% chlorhexidine gel) twice a week. At home, she disinfected the oral cavity after meals with H2O2, 0.2% chlorhexidine, and one or two applications of the same gel. This treatment controlled her pain, inflammation, and infectious episodes, but bone necrosis continued to spread.
In February, the patient presented with mandibular pain, a slight temperature (37.8℃), and signs of local inflammation with spontaneous emission of pus from one of the mandibular sites of necrosis. After cleaning and medication, therapy with amoxicillin and clavulanic acid (1 g 3 times a day for 10 days) and metronidazol (250 mg 3 times a day for 10 days) was prescribed. A facial computed tomography (CT) scan was performed without contrast medium, revealing multiple radiotransparent areas in both jaws, presumably a complication of BP therapy, and bilateral reactive laterocervical lymphadenopathy.
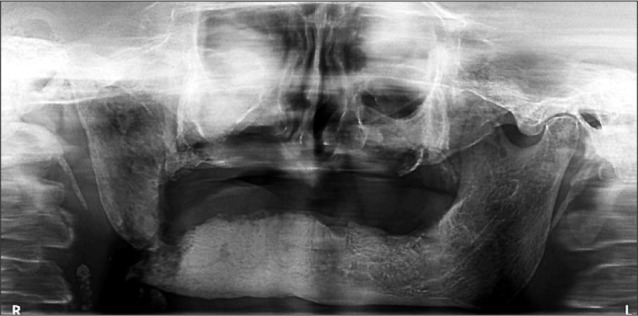
The patient did not return for follow-up the next week but presented urgently 15 days later. Local and general symptoms had worsened considerably, and she had a temperature of 39.1℃ with tachycardia, dysphagia, dyspnea, and tachypnea. Clinical examination showed extensive swelling of the face and neck; bright red extended skin, extremely painful to palpation; positive foveal sign; and incipient trismus. The mandible deviated to the right during opening, and oral examination showed major discharge of pus from the right mandible. A panoramic radiograph of the dental arches showed a full-thickness mandibular fracture.(Fig. 1)
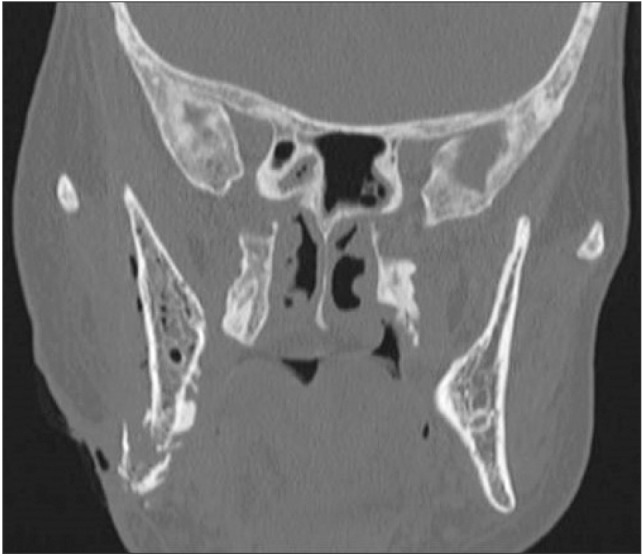
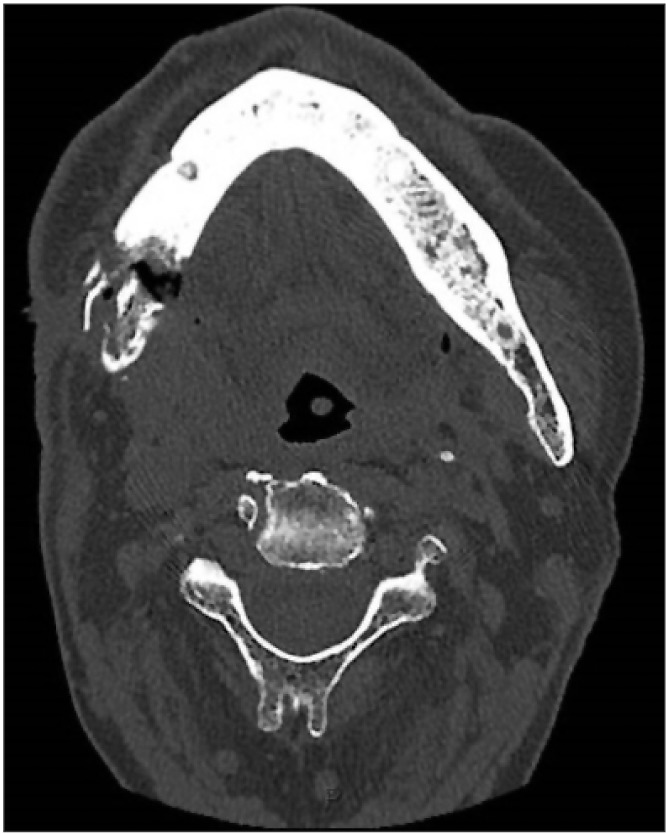
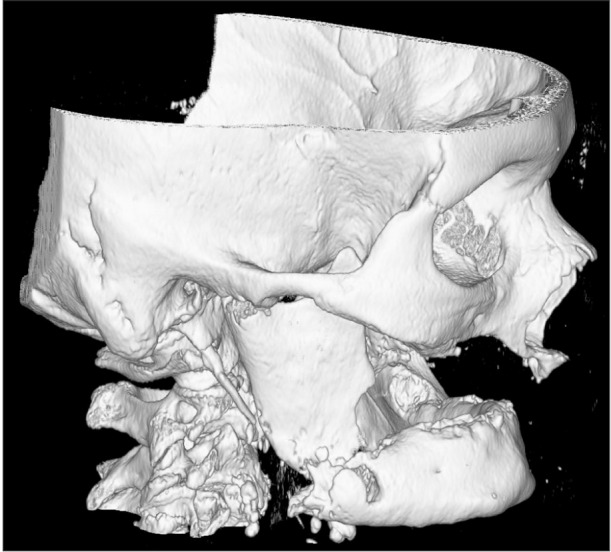
Due to systemic involvement, the patient was admitted to the ENT Department, where CT scan of the face, neck, and chest was performed without contrast medium, showing thickening of the soft tissue adjacent the inferior right hemimandibular surface; multiple bilateral abscesses in the upper cervical region, more evident on the right; and normal thoracic-mediastinal district, in addition to the right mandibular fracture.(Fig. 2, 3, 4) Blood chemistry revealed a white blood cell count of 42,100/mm3, C-reactive protein 31.7 mg/dL, and glycemia 183 mg/dL.
The patient was infused with metronidazole (1.5 g/day) and ceftriaxone (4 g/day), and the wounds were immediately drained surgically via bilateral cervicotomy. During the operation, necrosis of the superficial cervical fascia was observed, associated with thrombosis in the anterior jugular veins. These findings were compatible with necrotizing fasciitis. Empyema was observed in the submandibular space of the oral floor, adjacent to the fracture and extending to the anterior parapharynx. A swab was positive for Staphylococcus epidermidis, Pseudomonas aeruginosa, and Candida albicans. The jugular carotid and perilaryngeal-thyro-esophageal districts were free of empyema, though the tissues were diffusely edematous.
The patient was transferred to the intensive care unit, where antibiotic therapy was integrated with ciprofloxacin (500 mg twice a day i.v.) and imipenem (1.5 g/day) on the basis of an antibiogram. Daily local medication was continued with hydrogen peroxide diluted with normal saline and local antibiotic (ceftazidime).
After initial clinical improvement observed about 4 days after cervicotomy, the patient's condition deteriorated sharply, showing impaired cardiovascular function treated with calcium antagonists, beta blockers, antiplatelet agents, diuretics, electrolytes, and albumin (which is typically depleted in such conditions).
A CT scan showed an absence of empyema of the oral floor and cervical spaces due to drainage; however, the lungs showed diffuse signs of pneumonia with massive involvement on the left side. Despite systemic antibiotic treatment and repeated bronchial lavage, the patient's general condition deteriorated, culminating in septic shock. This was followed by kidney and liver failure and death due to multiple organ dysfunction on the 10th day.
Bone necrosis is a complication of therapy with BP and is related to the strength of the drug administered, duration of therapy6, and concomitance of trauma exposing bone of the jaws. Extraction of a tooth is often the triggering event. If dental treatment is necessary, BP should only be suspended when the risk of complications is high7.
Our patient has previously undergone surgery for breast cancer and was immediately treated with zoledronic acid. After seven months of BP therapy, she developed BRONJ. No dental events can explain the osteonecrosis. In the present case, progression of areas of necrosis led to jaw fracture, permitting entry of infective agents (usually Staphylococcus aureus and P. aeruginosa89) and their spread to the oral floor and submandibular space through sublingual tissue and the mylohyoid muscle. This gave rise to empyema involving the cervical fascia as necrotizing fasciitis10. The first therapies indicated were high doses of broad-spectrum antibiotics. The flora that generally cause this type of infection consist of resistant anaerobic and aerobic bacteria. The most suitable drugs for appropriate initial antibiotic therapy include penicillinase-resistant penicillin for streptococci and staphylococci, clindamycin or metronidazole for anaerobic gram-negative flora, and fluoroquinolones for Pseudomonas and aerobic and anaerobic bacteria11.
Other systemic diseases, conditions, and pharmacological therapies that contribute to poor general condition (diabetes, positivity for hepatitis B virus, hepatitis C virus, human immunodeficiency virus, smoking, alcohol abuse, obesity, cardiovascular disease, and immunosuppressant therapy) are fundamental factors in the evolution of BRONJ, infection, and fasciitis, as in the present case, and predispose the patient to a negative outcome. A good prognosis is often aided by early diagnosis, specific pharmacological therapy, and prompt surgical debridement 12. In the present case, the poor general condition of the patient made it impossible to limit spread of the infection.
Like other pathologies of odontogenic origin that can lead to severe conditions, such as necrotizing fasciitis and death, osteonecrosis of the jaw can be considered a possible source of local-regional infective complications.
Looking at the recent literature, there is another case of lethal cervical abscess following BRONJ. In that case, a 59-year-old female patient developed severe BRONJ with recurrent abscesses after oral osteoporosis therapy with alendronic acid. A subsequent submandibular abscess led to bacterial embolism of the left internal jugular vein, causing sepsis and death13.
Therefore, considering the possibility of an unfavorable patient course, the use of BP should be carefully monitored in order to prevent such severe complications. We therefore underline the importance of early diagnosis and appropriate medical and surgical treatment. Special attention should be paid to patients in poor general condition.
References
1. de Groen PC, Lubbe DF, Hirsch LJ, Daifotis A, Stephenson W, Freedholm D, et al. Esophagitis associated with the use of alendronate. N Engl J Med. 1996; 335:1016–1021. PMID: 8793925.

2. Marx RE. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg. 2003; 61:1115–1117. PMID: 12966493.

3. Marx RE, Sawatari Y, Fortin M, Broumand V. Bisphosphonate-induced exposed bone (osteonecrosis/osteopetrosis) of the jaws: risk factors, recognition, prevention, and treatment. J Oral Maxillofac Surg. 2005; 63:1567–1575. PMID: 16243172.

4. Woo SB, Hellstein JW, Kalmar JR. Narrative [corrected] review: bisphosphonates and osteonecrosis of the jaws. Ann Intern Med. 2006; 144:753–761. PMID: 16702591.
5. Otto S, Schreyer C, Hafner S, Mast G, Ehrenfeld M, Stürzenbaum S, et al. Bisphosphonate-related osteonecrosis of the jaws: characteristics, risk factors, clinical features, localization and impact on oncological treatment. J Craniomaxillofac Surg. 2012; 40:303–309. PMID: 21676622.
6. Lee SH, Chan RC, Chang SS, Tan YL, Chang KH, Lee MC, et al. Use of bisphosphonates and the risk of osteonecrosis among cancer patients: a systemic review and meta-analysis of the observational studies. Support Care Cancer. 2014; 22:553–560. PMID: 24203085.

7. Ruggiero SL, Woo SB. Biophosphonate-related osteonecrosis of the jaws. Dent Clin North Am. 2008; 52:111–128. ixPMID: 18154867.

8. Tsitsilonis S, Druschel C, Wichlas F, Haas NP, Schwabe P, Bail HJ, et al. Necrotizing fasciitis: is the bacterial spectrum changing? Langenbecks Arch Surg. 2013; 398:153–159. PMID: 22833058.

9. Greinwald JH Jr, Wilson JF, Haggerty PG. Peritonsillar abscess: an unlikely cause of necrotizing fasciitis. Ann Otol Rhinol Laryngol. 1995; 104:133–137. PMID: 7857015.

10. Tung-Yiu W, Jehn-Shyun H, Ching-Hung C, Hung-An C. Cervical necrotizing fasciitis of odontogenic origin: a report of 11 cases. J Oral Maxillofac Surg. 2000; 58:1347–1352. PMID: 11117681.

11. Lorenzini G, Picciotti M, Di Vece L, Pepponi E, Brindisi L, Vessio V, et al. Cervical necrotizing fasciitis of odontogenic origin involving the temporal region--a case report. J Craniomaxillofac Surg. 2011; 39:570–573. PMID: 22036666.
12. Roberson JB, Harper JL, Jauch EC. Mortality associated with cervicofacial necrotizing fasciitis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996; 82:264–267. PMID: 8884823.

13. Kaehling Ch, Streckbein P, Schmermund D, Henrich M, Burchert D, Gattenloehner S, et al. Lethal cervical abscess following bisphosphonate related osteonecrosis of the jaw. J Craniomaxillofac Surg. 2014; 42:1203–1206. PMID: 24680164.

Fig. 2
Computed tomography scan of the head; coronal view. Coronal view shows biphosphonate-related osteonecrosis lesions, fracture of right jaw and thickening of the soft tissue adjacent the inferior right hemimandibular surface in a 69-year-old woman with breast cancer history and treated with zoledronic acid for 23 administrations.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download