Abstract
Hemangioma is the most common benign tumor of a vascular origin, and is characterized by the abnormal proliferation of blood vessels. Intramuscular hemangioma (IMH) usually involves the skeletal muscles of the trunk or limbs, but rarely occurs in the head and neck region. This case report presents a patient with IMH showing multiple phleboliths in the buccal cheek. A 13-year-old boy was referred for the evaluation and management of painful swelling of the left cheek that had gradually increased in size over a 6 year duration. The examination revealed a palpable firm mass. Reddish-blue buccal mucosa color was observed with an aciniform shape. Preoperative magnetic resonance imaging (MRI) showed a vascular tumor in the left side adjacent to the buccinator and depressor orbicularis oris muscles. Surgical resection under general anesthesia was performed via the intraoral approach. The mass and phleboliths were extracted successfully. A histopathological examination confirmed the diagnosis of IMH. In conclusion, clinicians should be aware of the possibility of IMH in cases of a palpable mass with multiple nodules deep within the muscle in the buccal cheek. Among the several diagnostic tools, MRI provides essential information on the extent and surrounding anatomy of IMH.
Go to : 
Hemangioma is considered a benign vascular lesion of congenital origin, developing from abnormally differentiated blood vessels1. The exact cause is unknown, but excessive muscle contraction, repeated trauma to the lesion, and hormonal factors seem to play a role2. There is reportedly no gender predilection or racial factor3, and it is usually detected in the first 3 decades of life4.
Intramuscular hemangioma (IMH) is a relatively rare lesion, constituting less than 1% of all hemangioma cases, and is usually located in the skeletal muscles of the trunk or limbs5. IMH most frequently involves the pelvic region, but 10% to 15% occur in head and neck regions, generally in the masseter, sternomastoid, and trapezius muscles2. Among these, the masseter muscle is the most frequent location, comprising approximately 36% of all head and neck IMH cases6.
IMH in the masseter is described as a slowly enlarging mass with varied size, rubbery and relatively firm texture, and fluctuated with palpation perpendicular to the long axis of the muscle fibers5. It becomes prominent with muscle contraction, and more than a half of patients complain of associated pain with preauricular or buccal swelling7. Clinically, degree of the pain correlates with speed of expansion, pressure on surrounding anatomic structures, and thrombosis2. Sometimes, abrupt onset of facial palsy is reported, probably resulting from an enlarged lesion inducing pressure on the facial nerve8.
Standard radiographs are a simple diagnostic method for IMH since they can detect phleboliths, which are highly suggestive of hemangioma9. Other diagnostic imaging modalities such as computed tomography (CT), magnetic resonance imaging (MRI), and ultrasound can be used to enhance the accuracy of a preoperative diagnosis. Of these, MRI is considered the most reliable imaging tool for tissue characterization and identification of the extent of IMH10.
In this report, we presented a patient with IMH occurring in the left buccal cheek related to the adjacent muscles. Diagnosis was confirmed through clinical examination and preoperative MRI, and surgical resection was conducted. Also, optical microscope examination was conducted on the extracted specimen and internal phleboliths to determine their histopathologic features.
Written informed consent was obtained from the patient for publication of this report and any accompanying images.
Go to : 
A 13-year-old boy in good health was referred for evaluation and management of swelling of the left cheek. He showed a lesion suspicious of hemangioma on the left buccal cheek that first presented 6 years previous. He did not receive any treatment at the time due to his age and was recommended to return when the lesion became larger or induced pain. He re-visited our hospital since the mass had become larger and triggered intermittent pain.
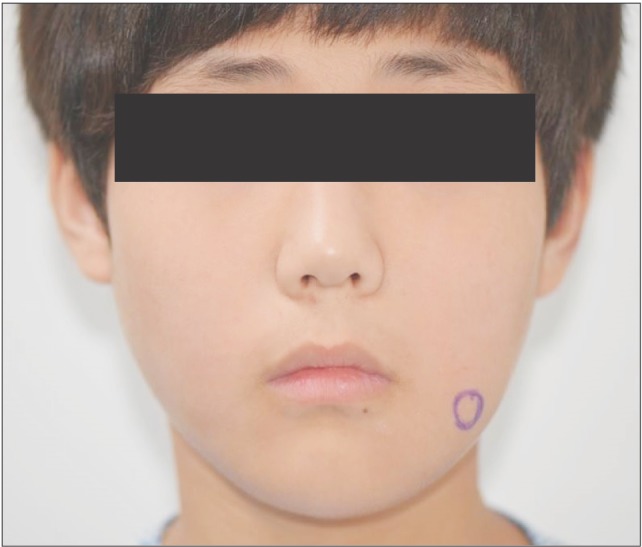
On clinical examination, he complained of mild swelling on the left buccal cheek and lower lip that was not outwardly conspicuous.(Fig. 1) The mass was firm and mobile on palpation and was located over the anterior portion of the mandibular angle. There were no bruits, pulsations, or lymphadenopathies.
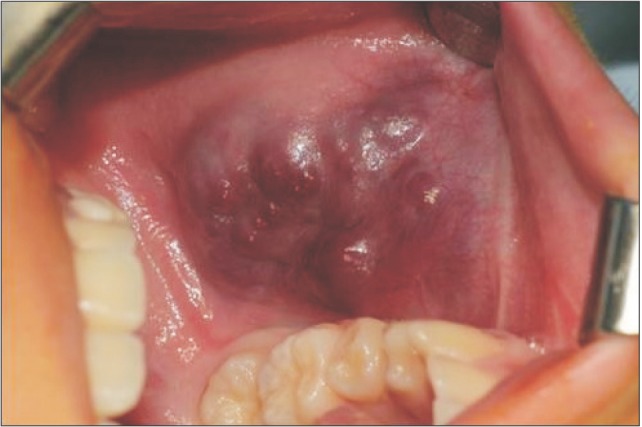
Intraorally, we found that the lesion was bulging under the buccal mucosa. The color of the buccal mucosa appeared reddish-blue, and several nodules looked aciniform.(Fig. 2)
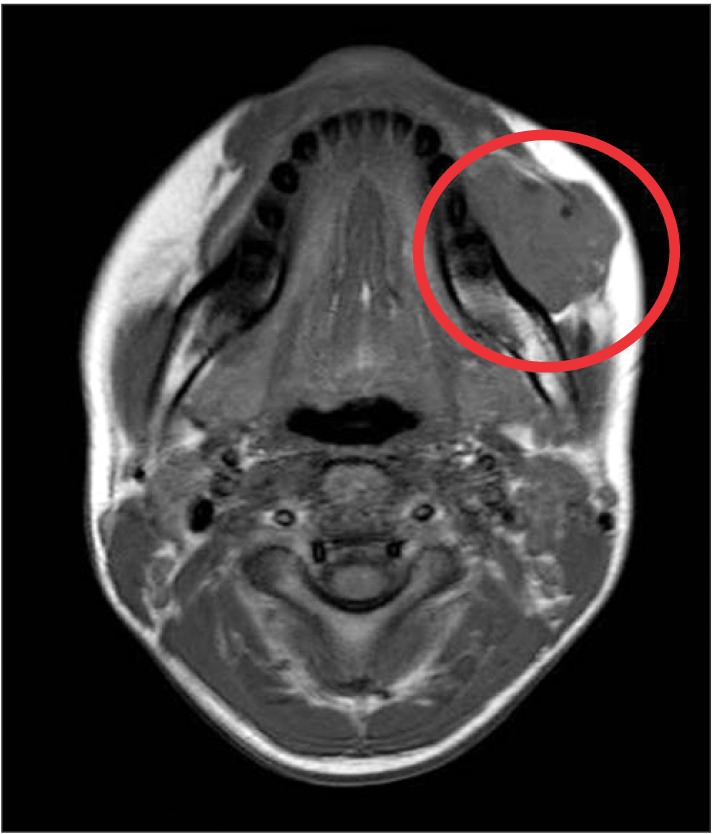
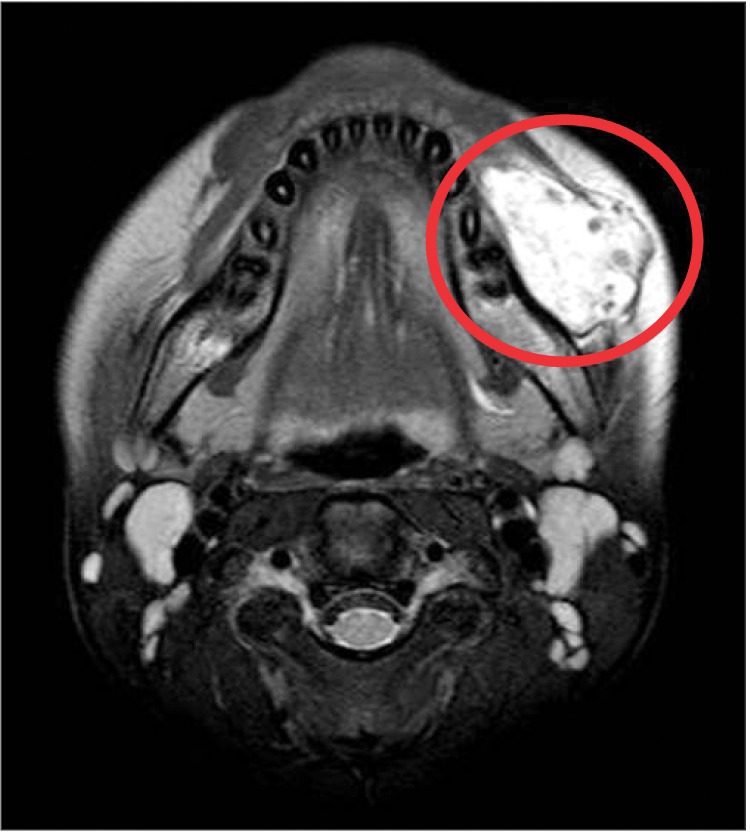
The patient had normal dentition and no other specific sign surrounding the teeth and mandible, including phleboliths on the image of plain radiograph. MRI indicated a mass on the left side of the buccal cheek, corresponding to the clinical examination.(Fig. 3, 4) The mass appeared with distinct borders as an isointense signal on T1-weighted and as a bright hyperintense signal on T2-weighted MRI, which was consistent with the presence of fluid. The inside of the mass contained several vacant spaces, which were assumed to be phleboliths related to the hemangioma. The mass was located beneath the buccinator and depressor anguli oris muscles.
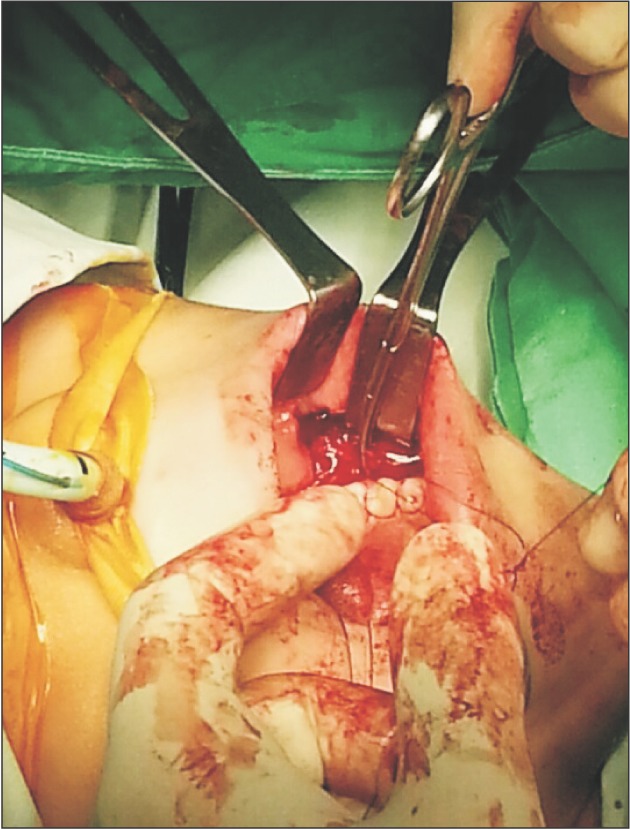
Under suspicion of hemangioma related to the adjacent muscles, we planned surgical excision of the lesion under general anesthesia. The surgical resection proceeded with minimal bleeding by ligating the feeding vessels and applying electrical coagulation to the minor vessels.(Fig. 5)
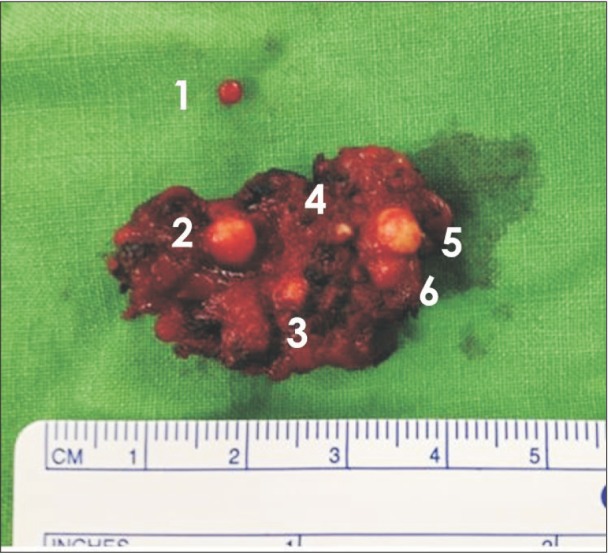
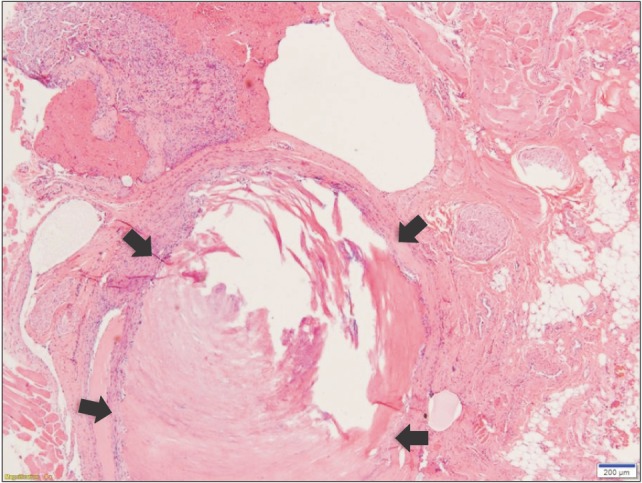
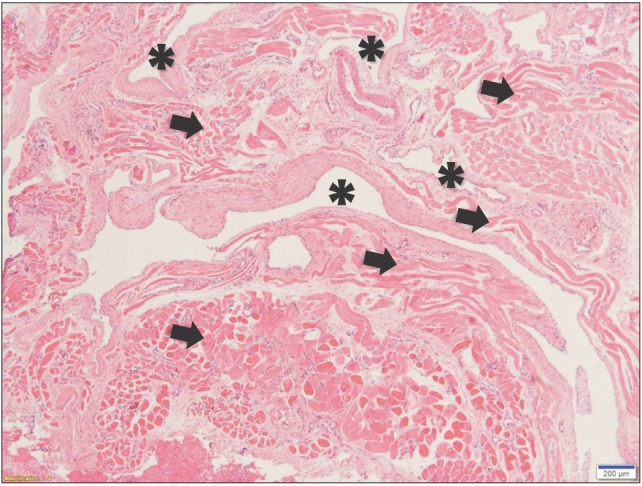
After surgery, we confirmed a total of 6 phleboliths with the extracted mass.(Fig. 6) Optical microscopy of the cut surface of the phlebolith suggested a concentric shape, corresponding to the growth mechanism of a phlebolith.(Fig. 7) The specimen was sent to the department of pathology for histopathological analysis.(Fig. 8) The result indicated multiple empty blood vessels and skeletal muscles around the vessels in the specimen. Skeletal muscles were observed with various patterns, forming round bundles or lining up along blood vessels. These patterns were the result of multiple blood vessels that had sprouted into the muscles and changed the patterns of muscle arrangement. According to previous findings, the final diagnosis of the specimen was established as IMH. There was no evidence of recurrence over 30 months of follow-up after surgery.
Go to : 
It is commonly known that skin overlying a hemangioma shows increased vascularity, giving the lesion reddish-blue discoloration or even hyperthermic change. Also, thrills, compressibility, or bruits can be present in hemangioma, though they are rare1. In many cases, the thickening and surrounding fibrosis of the superficial muscular layer around IMH hide these clinical signs5. The lack of clear clinical findings and the rare incidence of this lesion complicate the diagnosis. Definitive preoperative diagnosis has been reported in only about 8% of cases11. Also, other pathologic lesions are usually confused in the differential diagnosis, like neoplasms in parotid gland, benign muscular hypertrophy especially related to the masseter, or congenital cysts12.
Thus, various radiologic investigations should be performed to aid in diagnosis of IMH. Among them, CT scanning with contrast is extremely sensitive in detecting calcification and could be helpful for confirming presence of phleboliths and could give an indication of the anatomical origin of the tumor13. For better soft tissue detail, MRI provides images with good soft tissue definition of both normal anatomy and pathology. It is sensitive to blood flow within vessels and is used to determine the nature and extent of vascular malformations including hemangioma. Generally, IMH shows characteristically high signal intensity as a brighter lesion on T2-weighted images than T1-weighted images due to the increased free water present in stagnant blood in the vessels10.
In this case, we noted reddish-blue color change in the left buccal mucosa on clinical examination.(Fig. 2) Also, a bright lesion was seen on the left side nearby the masseter muscle on T2-weighted MRI, which is a typical sign of IMH. In virtue of these features, we diagnosed this lesion as IMH related to the masseter muscle before surgical treatment.
The incidence of phleboliths within IMH is approximately 25% of cases and usually causes no symptoms14. According to a previous study, tortuous vascular channels of the IMH produce thrombi by slowing of organized peripheral blood flow. First, calcification of the thrombus occurs, which then becomes the core of the phleboliths. The fibrinous component including platelets attaches to this core and is followed by calcification. Repetition of this course results in phlebolith growth5. Radiologically, the cores of phleboliths seem to be radiopaque or radiolucent, and a series of this process cause an onion-like or concentric rings pattern15.
Clinicians could confuse phleboliths in IMH close to the masseter muscle with sialoliths related to the parotid gland. Sialolithiasis can obstruct the parotid duct to cause sialadenitis with intermittent swellings and pain on the buccal cheek. In this condition, clinicians should remember the characteristics of phleboliths mentioned above and try to determine whether there is a neoplasm related to vascular malformation or a lesion in the parotid gland. Generally, phleboliths are smaller, more multiple, and more randomly located than sialoliths. Also, we found that sialoliths exist around the parotid duct and so have oval shapes. In addition to sialoliths, phleboliths from IMH should be distinguished from many other calcified lesions in the head and neck region2.
The treatment of hemangioma is based on clinical factors such as size, location, accessibility, depth of invasion, age, and cosmetic appearance. Many non-surgical treatments have been suggested to cure or control IMH; for example, cryotherapy, radiation therapy, arterial ligature, isolated embolization, sclerosing agents, or steroid injection. However, the results of these methods are controversial, so they are recommended only when surgical extirpation is contraindicated7. The best treatment for IMH is complete removal of the tumor including phleboliths with adjacent normal muscular tissue because of its infiltrative nature16. If needed, total excision of the masseter muscle has been recommended15. Rossiter et al.3 and Addante and Donavan17 agree with this idea that optimal treatment of IMH is complete surgical extirpation. Even with this surgical approach, minor feeding vessels and residual tumor can be responsible for recurrence. The local recurrence rate is approximately 18%, with 7% recurring more than once18.
Excision of a large lesion can result in severe hemorrhage. Preoperative arterial embolization and injection of sclerosing agents into the lesion are advised to solve this problem and help diminish blood loss19. Ligation of feeding vessels helps to minimize blood loss, and especially in cases of IMH in the lower portion of the masseter, tying the masseteric branch of the facial artery will help16. In addition, Ichimura et al.16 posited that securing a wide surgical field to expose the whole tumor and adjacent anatomic site could enable surgery with less bleeding.
Some approaches to surgical resection of IMH in the head and neck have been described. Superficial parotidectomy via preauricular incision provides good exposure but requires facial nerve dissection7. If the tumor exists anterior to the masseter muscle and close to the oral mucosa, an intraoral approach will be possible. This procedure is done with mucosal incision anterior to Stenson's duct and allows good visualization of the lesion and direct tumor excision with local bleeding control. Also, the patient can avoid any visible scar or facial nerve damage2.
Go to : 
References
1. Altuğ HA, Büyüksoy V, Okçu KM, Doğan N. Hemangiomas of the head and neck with phleboliths: clinical features, diagnostic imaging, and treatment of 3 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007; 103:e60–e64.

2. Mandel L, Surattanont F. Clinical and imaging diagnoses of intramuscular hemangiomas: the wattle sign and case reports. J Oral Maxillofac Surg. 2004; 62:754–758. PMID: 15170294.

3. Rossiter JL, Hendrix RA, Tom LW, Potsic WP. Intramuscular hemangioma of the head and neck. Otolaryngol Head Neck Surg. 1993; 108:18–26. PMID: 8437870.

4. Ingalls GK, Bonnington GJ, Sisk AL. Intramuscular hemangioma of the mentalis muscle. Oral Surg Oral Med Oral Pathol. 1985; 60:476–481. PMID: 3864109.

5. Kanaya H, Saito Y, Gama N, Konno W, Hirabayashi H, Haruna S. Intramuscular hemangioma of masseter muscle with prominent formation of phleboliths: a case report. Auris Nasus Larynx. 2008; 35:587–591. PMID: 18207684.

6. Odabasi AO, Metin KK, Mutlu C, Başak S, Erpek G. Intramuscular hemangioma of the masseter muscle. Eur Arch Otorhinolaryngol. 1999; 256:366–369. PMID: 10473832.

7. Righini CA, Berta E, Atallah I. Intramuscular cavernous hemangioma arising from the masseter muscle. Eur Ann Otorhinolaryngol Head Neck Dis. 2014; 131:57–59. PMID: 23845293.

8. Saeed WR, Kolhe PS, Smith FW, Murray GI. The ‘turkey wattle’ sign revisited: diagnosing parotid vascular malformations in the adult. Br J Plast Surg. 1997; 50:43–46. PMID: 9038514.

9. Biller HF, Krespi YP, Som PM. Combined therapy for vascular lesions of the head and neck with intra-arterial embolization and surgical excision. Otolaryngol Head Neck Surg. 1982; 90:37–47. PMID: 6806755.

10. Yonetsu K, Nakayama E, Yuasa K, Kanda S, Ozeki S, Shinohara M. Imaging findings of some buccomasseteric masses. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998; 86:755–759. PMID: 9868738.

11. Clemis JD, Briggs DR, Changus GW. Intramuscular hemangioma in the head and neck. Can J Otolaryngol. 1975; 4:339–346. PMID: 1139430.
12. Demir Z, Oktem F, Celebioğlu S. Rare case of intramasseteric cavernous hemangioma in a three-year-old boy: a diagnostic dilemma. Ann Otol Rhinol Laryngol. 2004; 113:455–458. PMID: 15224828.

13. Yonetsu K, Nakayama E, Miwa K, Tanaka T, Araki K, Kanda S, et al. Magnetic resonance imaging of oral and maxillofacial angiomas. Oral Surg Oral Med Oral Pathol. 1993; 76:783–789. PMID: 8284086.

14. Osada K, Yoshihara T, Itoh M. Intramasseter hemangiomas: a case report. J Otolaryngol. 2000; 29:166–169. PMID: 10883831.
15. Zengin AZ, Celenk P, Sumer AP. Intramuscular hemangioma presenting with multiple phleboliths: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013; 115:e32–e36.

16. Ichimura K, Nibu K, Tanaka T. Essentials of surgical treatment for intramasseteric hemangioma. Eur Arch Otorhinolaryngol. 1995; 252:125–129. PMID: 7662343.

17. Addante RR, Donovan MG. Right facial mass. J Oral Maxillofac Surg. 1994; 52:1061–1065. PMID: 8089793.

18. Allen PW, Enzinger FM. Hemangioma of skeletal muscle. An analysis of 89 cases. Cancer. 1972; 29:8–22. PMID: 5061701.
19. Van Doorne L, De Maeseneer M, Stricker C, Vanrensbergen R, Stricker M. Diagnosis and treatment of vascular lesions of the lip. Br J Oral Maxillofac Surg. 2002; 40:497–503. PMID: 12464208.

Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download