Abstract
Squamous cell carcinoma of the buccal mucosa has an aggressive nature, as it grows rapidly and penetrates well with a high recurrence rate. If cancers originating from the buccal mucosa invade adjacent anatomical structures, surgical tumor resection becomes more challenging, thus raising specific considerations for reconstruction relative to the extent of resection. The present case describes the surgical management of a 58-year-old man who presented with persistent ulceration of the mucosal membrane and a mouth-opening limitation of 11 mm. Diagnostic imaging revealed a buccal mucosa tumor that had invaded the retroantral space upward with involvement of the anterior border of the masseter muscle by the lateral part of the tumor. In this report, we present the surgical approach we used to access the masticator space behind the maxillary sinus and discuss how to manage possible damage to Stensen's duct during resection of buccal mucosa tumors.
Buccal mucosa cancer primarily occurs along the occlusal plane and is characterized by pain and ulceration, which are usually accompanied by a buccal mass. Squamous cell carcinoma (SCC) of the buccal mucosa is rare and accounts for approximately 10% of all oral cancers12. In an investigation of the development sites of oral SCC in Koreans, the buccal mucosa was reported to be the fourth most common site following the mandible, tongue, and maxilla3.
Buccal mucosa SCC is known to grow more rapidly and penetrate well with a higher recurrence rate than oral SCCs at other sites. Therefore, buccal mucosa SCC requires careful treatment even at early stages4. The buccal mucosa is anatomically connected to the vestibule of the maxilla and mandible, retromolar trigone, and masseter muscle. Thus, buccal mucosa cancer can invade adjacent structures, such as upper and lower jaws, masticatory muscles, and cheeks, often rendering surgical resection and reconstruction more challenging, particularly when the cancer invades the masticator space; furthermore, it is even more complicated when mouth opening is limited. Following surgical resection of the tumor, appropriate reconstruction is necessary to minimize functional and esthetic issues.
Here we present a case report to share our experience in the management of a patient with buccal mucosa SCC infiltrating into the masticator space. The written informed consent was obtained from the patient.
A 58-year-old man was referred to our outpatient department with complaints of a gradually worsening trismus and painful ulcerated wound in the right buccal mucosa that failed to heal since the past 1 year. The patient was on medications for hypertension and coronary artery thrombosis and had no other specific systemic disease. Clinically, the maximum mouth opening was 11 mm, ulceration was observed in the left buccal mucosa, and a firm mass could be palpated on the skin of the left cheek. No palpable cervical lymphadenopathy was observed. The patient underwent workup for suspected malignancy of the buccal mucosa. Following imaging tests, an incisional biopsy of the left buccal mucosa was performed, which confirmed the diagnosis of SCC. Computed tomography (CT) showed a buccal mucosa tumor that extended superiorly to the retroantral space and destructed the lateral wall of the maxillary sinus, inferiorly to the retromolar trigone, and laterally to the buccinator muscle and anterior border of the masseter muscle, with no evidence of cervical lymph node metastasis.(Fig. 1) No evidence of regional or distant metastasis was found based on positron emission tomography-CT and other test results.
Surgical strategy was as follows: because the tumor extended to the masticator space behind the maxillary sinus and trismus was present, surgical approach to this restricted tumor became more challenging; thus, we used the mandibular swing technique combined with a modified Weber-Ferguson incision to approach the tumor. Accordingly, mandibulotomy was performed in the region between #33 and #34 after lower-lip splitting, and the incision was extended to the left submandibular region. The upper-lip incision was extended to the outer rhinotomy to a level 1 cm below the left medial canthus. The skin incision was continued into an intraoral vestibular incision, and the upper and lower cheek flaps were elevated after performing subperiosteal dissection in the maxilla and mandible. Using this approach, wide exposure of the infratemporal space required for surgical resection was obtained.
En bloc resection was performed with a 1-cm safety margin because the buccal mucosal tumor extended to the retroantral space, retromolar trigone, and masseter muscle beyond the buccinator muscle, with suspicious invasion of the subcutaneous layer of the cheek. The regions of the maxillary sinus adjacent to the tumor from the coronoid process of the mandible to the lower region of the retromolar trigone and the cheek skin were included in the single mass.(Fig. 2) In addition, a prophylactic neck dissection was performed, although no evidence of cervical lymph node metastasis was noted on preoperative imaging. To evaluate the tumor margin and regional neck metastasis at the time of surgery, frozen section diagnosis was examined, which was negative for malignancy.
After surgical resection, the through-and-through defect of the orofacial region was reconstructed using a double-paddled latissimus dorsi free flap. An end-to-end anastomosis was performed between the superior thyroid artery and thoracodorsal artery, and an end-to-side anastomosis was performed between a branch of the internal jugular vein and thoracodorsal vein.
The patient showed good postoperative course without any abnormal clinical and laboratory findings. However, a yellowish discharge through the neck area drain was observed from the 5th postoperative day and continued for several days. The patient complained of dull pain in the left preauricular region, but the flap seemed well perfused on daily doppler check with no signs of suspicious postoperative inflammation. We considered the pain to be salivary retention and digestion of tissue by the leaked saliva oozing from the damaged Stensen's duct. Therefore, we performed Stensen's duct relocation on the 12th postoperative day. A severed duct was identified and a cut-down tube was inserted into the duct after lumen patency was established, and the tube was secured in the upper labial mucosa such that saliva could be discharged into the oral cavity. A pressure dressing was applied on the left facial region after the ductoplasty. Salivary secretion through the tube resumed, the patient's pain resolved, and the yellowish discharge stopped.(Fig. 3) The cut-down tube was removed after completion of postoperative radiotherapy.
No postoperative complications such as infection of the surgical site or necrosis of the flap were reported until discharge. No issues related to pronunciation or mastication were noted, and the patient could start a regular diet. Trismus was resolved with a maximum mouth opening of 34 mm. Moreover, at the donor site, no specific functional shoulder deficit was observed.(Fig. 4) Based on the final pathology report, the resection margin closed at the medial and posterior regions of the mass with no regional lymph node metastasis; subsequently, the planned postoperative radiotherapy was performed without any further resection.
Buccal mucosa SCC is known to be aggressive in nature compared with oral cancers at other sites. It has been reported to have poor local control and 5-year cause-specific survival rates in early-stage carcinomas compared with those in the oral cavity, tongue, and mouth floor4. The reported recurrence rate of buccal mucosa SCC is 30% to 80%5678910. Thus, acquiring an adequate surgical resection margin is crucial during surgical resection. In early stages when the cancer is limited to the buccal mucosa and submucosal region, it is recommended to include the buccinator muscle in the resection margin. If the lesion invades beyond the submucosal region to the buccinator muscle, resection including the buccinator space should be considered. When positive margins are reported in the subcutaneous tissue, wide resection including the skin should be performed. Prophylactic neck dissection is recommended, even for 2- to 4-cm-sized tumors because advanced mucosal cancers are more likely to develop latent metastasis even with no clinical regional lymph node metastasis11. Local control has been reported to improve with postoperative radiotherapy in the early stage of buccal mucosal cancers12.
The masticator space contains the medial and lateral pterygoid muscle, masseter muscle, temporalis muscle, vertical ramus, and temporomandibular joint. The third division of the trigeminal nerve and its branches passes through this space, and the internal maxillary artery with its branches runs through this space and enters the pterygopalatine fossa13. Several spaces are in contact with the masticator space, such as the buccal and retroantral spaces anteriorly, parapharyngeal space medially, and parotid space laterally.
Several surgical approaches have been introduced for the resection of tumors in this space. The Weber-Ferguson incision and its modifications have been introduced as an anterior approach to the maxilla, but these methods are disadvantageous as maxillary separation is blindly performed, and exposure of the posterior maxilla is limited. The facial incision and bony defect are not esthetic and have the disadvantage of having to sacrifice several deeper structures. The Conley's lateral approach of extending the preauricular incision to the neck with a second submandibular incision has been proposed but has the disadvantages of facial incision and bony defect along with the sacrifice of inner deep structures14. Later, Castro et al.13 revised this method and published an approach to malignant tumors of the masticator space through a preauricular incision and transcervical incision; however, this method also has the burden of bypassing facial nerve trunks to access inner neoplasms. Dingman and Conley15 introduced an inferior approach through the submandibular incision that included midline lip splitting and posterior extension to the mastoid process. After horizontal osteotomy of the ascending ramus of the mandible, outward retraction of the superior portion, and downward retraction of the inferior portion, direct access to the pterygomaxillary region could be possible14. Spiro et al.16 reported that the mandibular “swing” approach, including lip-splitting incision extended to the mentum-to-mastoid portion, and median mandibulotomy with paralingual extension enables adequate exposure for the resection of oropharyngeal tumors.
In our case, a malignant neoplasm developing in the buccal mucosa had invaded the masticator space, which was accompanied by trismus, making the surgical approach more challenging. Therefore, we adopted previous surgical approaches and modified them by placing emphasis on obtaining adequate visual field of the masticator space. A modified Weber-Ferguson incision was made with no lateral eyelid extension in the maxilla along with lower-lip splitting that extended down to the submandibular region using a continued intraoral vestibular incision. Subsequently, after performing mandibulotomy, the upper cheek flap of the maxilla and the lower cheek flap of the mandible were elevated outward. A direct view of the lateral and posterior aspects of the maxilla and anatomical structures located medial to the ramus of the mandible could be acquired using this approach. This allowed complete surgical resection with an adequate safety margin, reduced operation time, and better ability to control bleeding from the internal maxillary artery and its branches or pterygoid plexus. From an esthetic viewpoint, this approach could minimize scars on the midface by not extending the lateral eyelid incision; further, scarring in the submandibular region is hardly visible in the natural head position. Moreover, because the incision was made along the patient's crease line, most of the scars were hidden in the wrinkles.
Various flaps that can be used for the through-and-through defect of the oral cavity after surgical resection have been proposed, including the radial forearm free, deltopectal, pectoralis major, latissimus dorsi free, transverse rectus abdominis myocutaneous, and trapezius myocutaneous flaps17181920. The latissimus dorsi free flap is a richly vascularized muscle with the largest potential surface area, providing adequate bulk and coverage for any defect in the oral and maxillofacial region. Moreover, this flap has the advantage that it allows primary closure of the donor site, which may prevent additional morbidity19. If a folded flap is covered with an orofacial defect, it may appear less esthetic because of its large volume, but as the volume of the flap decreases over time, the outcomes become more esthetic1718. A previous study reported an approximately 20% volume reduction in cases of reconstruction with a latissimus dorsi flap after tumor ablation as well as additional fat and muscle atrophy in patients receiving postoperative radiotherapy19. Therefore, postoperative flap atrophy, such as intentional overcorrection during flap reconstruction, must be considered.
The Stensen's duct starts from the anterior portion of the parotid gland and runs anteriorly across the anterior border of the masseter muscle. At the level of the masseter muscle, the duct runs inward, piercing through the buccal fat pad and buccinator muscle to produce a papilla orifice in the buccal mucosa at the level of the maxillary second molar. The length of the duct is approximately 7.0 cm, and its location can be estimated by drawing a line connecting the tragus to the mid-portion of the upper lip21.
Parotid gland and duct injuries are typically managed by repair of the injury, putting a stent into the duct, and placing a pressure dressing. If the stent is inserted after the parotid duct is damaged, it is usually removed after 1 week. When there is severe damage to the gland and its duct, ligation of the proximal portion of the duct is recommended, and the gland will gradually undergo atrophy22. It is known that strictures, cheek swelling, fistulas, and obstructive sialadenitis may occur if the duct is cut without repair23.
The aforementioned management principles for parotid duct injuries can be similarly applied after the resection of benign or malignant neoplasms of the buccal mucosa. Deygles et al.24 reported successful results by inserting an intravenous catheter into the parotid duct and activating salivary drainage for 1 week after surgical resection of a right buccal mucosal fibroepithelial hyperplasia. Longo et al.25 reported that after resection of SCC in cheek mucosa, an angiocatheter was inserted and removed at 10 days, which preserved parotid gland function without any complications. In addition, Mehta et al.23 reported that the incidence of sialocele and parotitis in the early postoperative period was significantly reduced by intravenous catheter cannulation and rerouting of the parotid duct after surgical resection of buccal mucosa cancer.
We also confirmed that the yellowish drainage had stopped and the clinical symptoms were much better after the insertion of a cut-down tube into Stensen's duct and reactivating parotid salivary flow, followed by a pressure dressing. Therefore, we recommend including a process to preserve parotid function during the surgical planning stage if the resection margin of the buccal mucosa includes certain sections of the parotid duct.
In conclusion, buccal mucosa SCC is aggressive, grows rapidly, and has a high recurrence rate; therefore, careful treatment is required even if the cancer is at an early stage. If a tumor of ≥T2 is identified, prophylactic neck dissection is recommended, and postoperative radiotherapy may be helpful for local control. In the present case, the modified Weber-Ferguson incision of the maxilla combined with the mandibular swing approach facilitated adequate exposure of the lesion in the masticator space, was a time-saving procedure, and provided acceptable esthetic outcomes after the surgery. Nevertheless, it is also necessary to consider preservation of parotid glandular function due to damage to the Stensen's duct during surgery due to buccal mucosal cancers by performing a simple Stensen's ductoplasty procedure.
References
1. Shah JP, Cendon RA, Farr HW, Strong EW. Carcinoma of the oral cavity. factors affecting treatment failure at the primary site and neck. Am J Surg. 1976; 132:504–507. PMID: 1015542.
2. Vegers JW, Snow GB, van der Waal I. Squamous cell carcinoma of the buccal mucosa. A review of 85 cases. Arch Otolaryngol. 1979; 105:192–195. PMID: 426706.

3. Kuk SK, Kim BK, Yoon HJ, Hong SD, Hong SP, Lee JI. Investigation on the age and location of oral squamous cell carcinoma incidence in Korea. Korean J Oral Maxillofac Pathol. 2015; 39:393–402.

4. Lin CS, Jen YM, Cheng MF, Lin YS, Su WF, Hwang JM, et al. Squamous cell carcinoma of the buccal mucosa: an aggressive cancer requiring multimodality treatment. Head Neck. 2006; 28:150–157. PMID: 16200628.

5. Bloom ND, Spiro RH. Carcinoma of the cheek mucosa. A retrospective analysis. Am J Surg. 1980; 140:556–559. PMID: 7425239.
6. Conley J, Sadoyama JA. Squamous cell cancer of the buccal mucosa. A review of 90 cases. Arch Otolaryngol. 1973; 97:330–333. PMID: 4573067.

7. Lapeyre M, Peiffert D, Malissard L, Hoffstetter S, Pernot M. An original technique of brachytherapy in the treatment of epidermoid carcinomas of the buccal mucosa. Int J Radiat Oncol Biol Phys. 1995; 33:447–454. PMID: 7673032.

8. Pop LA, Eijkenboom WM, de Boer MF, de Jong PC, Knegt P, Levendag PC, et al. Evaluation of treatment results of squamous cell carcinoma of the buccal mucosa. Int J Radiat Oncol Biol Phys. 1989; 16:483–487. PMID: 2921152.

9. Strome SE, To W, Strawderman M, Gersten K, Devaney KO, Bradford CR, et al. Squamous cell carcinoma of the buccal mucosa. Otolaryngol Head Neck Surg. 1999; 120:375–379. PMID: 10064641.

10. Urist MM, O'Brien CJ, Soong SJ, Visscher DW, Maddox WA. Squamous cell carcinoma of the buccal mucosa: analysis of prognostic factors. Am J Surg. 1987; 154:411–414. PMID: 3661845.

11. Hakeem AH, Pradhan SA, Tubachi J, Kannan R. Outcome of per oral wide excision of T1-2 N0 localized squamous cell cancer of the buccal mucosa--analysis of 156 cases. Laryngoscope. 2013; 123:177–180. PMID: 22952001.

12. Sieczka E, Datta R, Singh A, Loree T, Rigual N, Orner J, et al. Cancer of the buccal mucosa: are margins and T-stage accurate predictors of local control? Am J Otolaryngol. 2001; 22:395–399. PMID: 11713724.

13. Castro J, Likhterov I, Mehra S, Bassiri-Tehrani M, Scherl S, Clain J, et al. Approach to en bloc resection and reconstruction of primary masticator space malignancies. Laryngoscope. 2016; 126:372–377. PMID: 26526821.

14. Pogrel MA, Kaplan MJ. Surgical approach to the pterygomaxillary region. J Oral Maxillofac Surg. 1986; 44:183–187. PMID: 3456438.

15. Dingman DL, Conley J. Lateral approach to the pterygomaxillary region. Ann Otol Rhinol Laryngol. 1970; 79:967–969. PMID: 5506040.

16. Spiro RH, Gerold FP, Strong EW. Mandibular "swing" approach for oral and oropharyngeal tumors. Head Neck Surg. 1981; 3:371–378. PMID: 6263826.

17. Welvaart K, Caspers RJ, Verkes RJ, Hermans J. The choice between surgical resection and radiation therapy for patients with cancer of the esophagus and cardia: a retrospective comparison between two treatments. J Surg Oncol. 1991; 47:225–229. PMID: 1713631.

18. Hiraki A, Yamamoto T, Yoshida R, Nagata M, Kawahara K, Nakagawa Y, et al. Factors affecting volume change of myocutaneous flaps in oral cancer. Int J Oral Maxillofac Surg. 2016; 45:1395–1399. PMID: 27170618.

19. Li BH, Jung HJ, Choi SW, Kim SM, Kim MJ, Lee JH. Latissimus dorsi (LD) free flap and reconstruction plate used for extensive maxillo-mandibular reconstruction after tumour ablation. J Craniomaxillofac Surg. 2012; 40:e293–e300. PMID: 22377010.

20. Yang ZH, Zhang DM, Chen WL, Wang YY, Fan S. Reconstruction of through-and-through oral cavity defects with folded extended vertical lower trapezius island myocutaneous flap. Br J Oral Maxillofac Surg. 2013; 51:731–735. PMID: 24090763.

21. Haggerty CJ, Laughlin RM. Atlas of operative oral and maxillofacial surgery. Ames: Wiley-Blackwell Publishing;2015.
22. Van Sickels JE. Management of parotid gland and duct injuries. Oral Maxillofac Surg Clin North Am. 2009; 21:243–246. PMID: 19348990.

23. Mehta S, Agrawal J, Dewan AK, Pradhan T. Parotid duct relocation in buccal mucosa cancer resection. J Craniofac Surg. 2014; 25:1746–1747. PMID: 25162543.

24. Deygles C Jr, Medeiros R, Carvalho EJ, Carvalho AA. Catheterization of Stenon's duct for surgical excision of oral fibroepithelial hyperplasia. Braz J Otorhinolaryngol. 2012; 78:141. PMID: 22392253.
25. Longo B, Germano S, Laporta R, Belli E, Santanelli F. Stensen duct relocation after cheek mucosa tumor resection. J Craniofac Surg. 2012; 23:e250–e251. PMID: 22627447.

Fig. 1
A. Preoperative frontal view. Note the dimple on the left cheek, which raised suspicion of a subcutaneous layer invasion. B. Contrast computed tomography revealed a tumor extending into the masticator space and destructing the left maxillary sinus wall. C. Along with the buccal mucosa tumor, magnetic resonance imaging revealed a laterally extending tumor in the region (arrow) of the subcutaneous layer.
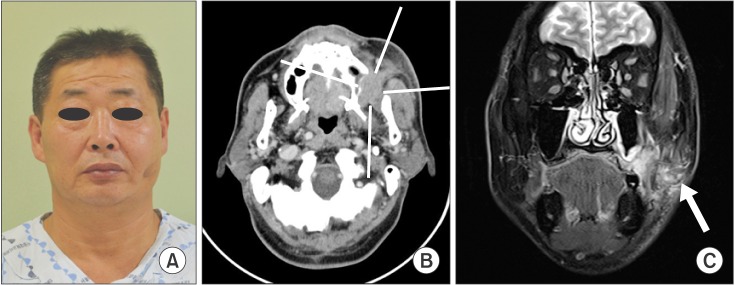
Fig. 2
Surgical approach and mass resection procedure. A modified Weber-Ferguson incision, without lateral extension on the maxilla combined with lower-lip splitting with mandibulotomy, was made and then a cheek flap was laterally reflected to provide sufficient exposure of the lateroposterior aspect of the maxillary sinus.
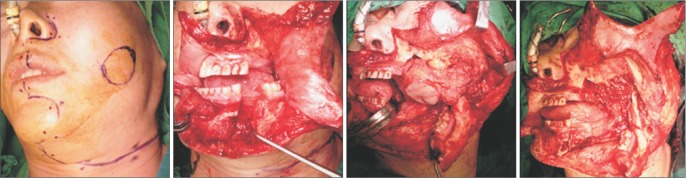
Fig. 3
A. A cut-down tube was inserted into the proximal stump of the severed Stensen's duct. B. Salivary secretion resumed through the tube, which was secured in the oral cavity, and clinical symptoms improved.
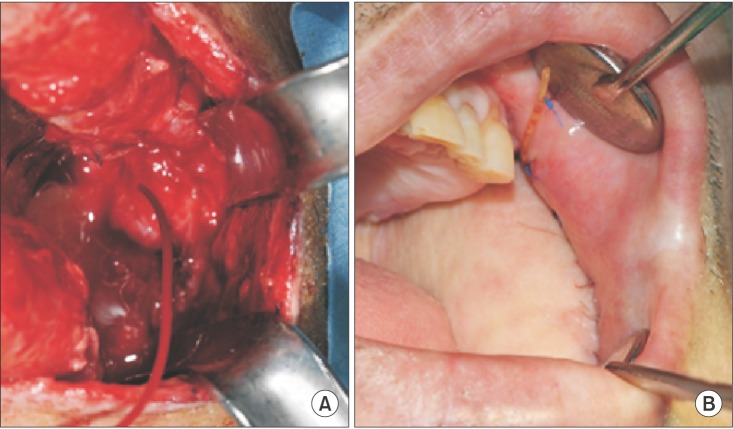
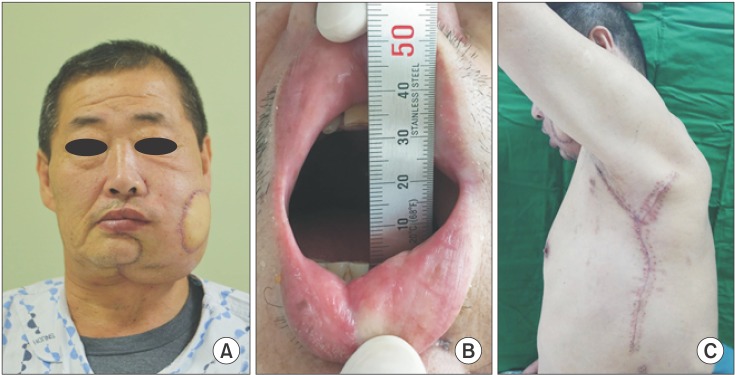
Fig. 4
Frontal view 1 month after surgery. Facial asymmetry was observed owing to the double-paddled reconstruction with a latissimus dorsi free flap, but gradual atrophy of the flap is expected. B. The maximum mouth opening measured at 1 month after surgery. Trismus was resolved. C. The patient was able to freely move his left shoulder and did not complain of discomfort at the donor site.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download